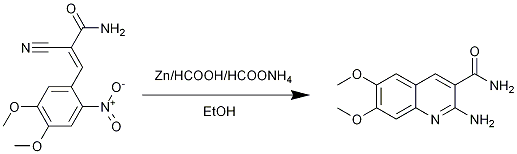
Quinoline synthesis via zinc/formic acid reductive cyclization
SyntheticPage 244
DOI:
Submitted: March 2, 2006, published: March 7, 2006
Authors
Vedran Hasimbegovic (vedran.hasimbegovic@gmail.com)
A contribution from
Chemicals
Formic acid 98% (Labassco)
Hydrochloric acid 37% (Scharlau)
Zinc Chem. Rein. (Reidel/De Haän AG Seelze-Hannover)
Ethanol 99.7 vol % (Solveco)
Hydrochloric acid 37% (Scharlau)
Zinc Chem. Rein. (Reidel/De Haän AG Seelze-Hannover)
Ethanol 99.7 vol % (Solveco)
Procedure
Zinc (4 g, 61.9 mmol) was added to HCl (5%, 50 mL) and the mixture was stirred for 5 minutes (hydrogen evolution). The liquid was decanted and the metal washed with distilled water (2 x 25 mL) followed by ethanol (20 mL). The hence activated zinc was added, in one portion, to a suspension of 2-cyano-3-(4,5-dimethoxy-2-nitrophenyl)acrylamide [[236|Page 236]] (1.2 g, 4.32 mmol), ethanol (15 mL), HCOOH (85%, 15 mL) and ammonium formate (0.3 g, 4.76 mmol). The bright-yellow mixture was refluxed for 4 h and then allowed to stir over weekend at room temperature. After this time a thick, pale-yellow paste was obtained. Enough ethanol was added so that the mixture could be vacuum filtered; dark-brown filtrate was discarded. While under vacuum, the yellow filter cake was treated with 98% HCOOH until it finally became white and gray (only unreacted zinc and its salts remaining). Yellow filtrate, containing the product, was first treated with equal volume ice-cold water, and then basified with 10 % NaOH until yellow percipitate apeared. The solid was filtered, washed with cold i-PrOH and dried in oven to give 0.39 g (43.5 %) 2-amino-6,7-dimethoxyquinoline-3-carboxamide as mat yellow crystals, m.p: 260-261?C, (decomp.).
Author Comments
The problem was to find a reduction system which would efficiently reduce the aromatic nitro to amine (thus furnishing 6-exo-dig) while at the same time be tolerated by nitrile and amide functions. This prompted some experimentation; first attempt was made following the original patent-method [1] in which the authors used iron/acetic acid/DMF. In my hands this resulted in intracable tar which was impossible to dissolve in accessible NMR solvents. On the other hand, Zn/formic acid gave desired quinoline in 43.5% yield with easy workup. It is recommended to first remove all formic acid under vacuum before adding ethanol and filtering, as the product later proved quite soluble in formic acid, though nothing precipitated upon addition of water/10% NaOH to the dark-brown filtrate. Less zinc, especially dust, can be used, I used quite an excess since only granulate was available at the moment. Product-spot is visible only under 254 nm UV, while the starting-material-spot is visible only at 366 nm UV.
Data
1H NMR 300 MHz (d-acetic acid): 8.72 (1H, s, pyridinyl), 7.16 (2H, d, phenyl), 4.02 (3H, s, methoxy), 3.93 (3H, s, methoxy).
TLC with EtOAc:IPA (3:2) as eluent: Rf(2-cyano-3-(4,5-dimethoxy-2-nitrophenyl)acrylamide): 0.93, Rf(product): 0.22 (fluorescent)
Other References
[1]: Patent; Pfizer Inc.; FR 2225166; 1974; DE 2418498