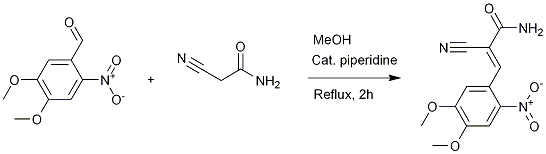
Knoevenagel condensation of 2-cyanoacetamide with 6-nitroveratraldehyde
SyntheticPage 236
DOI:
Submitted: January 31, 2006, published: February 1, 2006
Authors
Vedran Hasimbegovic (vedran.hasimbegovic@gmail.com)
A contribution from
Chemicals
2-propanol, analytical grade (Scharlau)
Methanol, analytical grade (Scharlau)
2-cyanoacetamide, 99% (Aldrich)
4,5-dimethoxy-2-nitro-benzaldehyde, technical grade (Aldrich)
Piperidine (Labassco)
Methanol, analytical grade (Scharlau)
2-cyanoacetamide, 99% (Aldrich)
4,5-dimethoxy-2-nitro-benzaldehyde, technical grade (Aldrich)
Piperidine (Labassco)
Procedure
To a slurry of 6-nitroveratraldehyde (5 g, 23.7 mmol) in methanol (50 mL) there was added 2-cyanoacetamide (2.2 g, 26.2 mmol), followed by piperidine (10 drops). The mixture was heated to reflux for 2 h, then cooled in an ice bath and finally filtered with suction. The intense-yellow crystals were washed with i-PrOH (30 mL) and air-dried to yield 5.6 g (100 %) of 2-cyano-3-(4,5-dimethoxy-2-nitrophenyl)acrylamide: mp 259-260 °C (literature[1]: 265-266°C)
Author Comments
The reaction was done twice, first time on 2.5 mmol scale yielding only 60%. The initially difficult-to-stir slurry becomes somewhat stirrable once heated up. TLC was run with EtOAc pre-treated with a couple of drops glacial acetic acid. This proved to be a nice trick in order to eliminate the “tail” behind the product-spot (which appeared with plain EtOAc). The starting material contained impurities (two very weak spots), the given Rf value corresponds to the most intense one.
Data
1H NMR 300 MHz (DMSO): 8.53 (1H, s CH=C), 7.93 (2H, d, amide), 7.81 (1H, s, ArH), 7.50 (1H, s, ArH), 3.95 (3H, s, OCH3), 3.94 (3H, s, OCH3) TLC with EtOAc (containing 5 drops AcOH) as eluent: Rf(6-nitroveratraldehyde): 0.9 (3 spots), Rf(product): 0.72 (one spot).
Lead Reference
[1]: Journal of Medicinal Chemistry, 1980, 23, 262-269
Other References
Patent; Pfizer Inc.; FR 2225166; 1974; DE 2418498; Chem.Abstr.; 82; 73015.
Keywords
Comments
The method is really good! Is the E/Z ratio 100% E?
By Mladen Litvic on February 24, 2006
Various Knoevenagel condensation reactions are reported occur in water. For eg.
Tetrahedron Letters, Volume 46, Issue 38, 19 September 2005, Pages 6453-6456
I think rather than using toxic solvent such as methanol, this reaction can be tried in water.
By Sandeep Sundriyal on January 8, 2007