A rapid, one-flask alpha halogenation of a ketone and subsequent haloketone amination
SyntheticPage 233
DOI:
Submitted: December 17, 2005, published: December 18, 2005
Authors
Vedran Hasimbegovic (vedran.hasimbegovic@gmail.com)
A contribution from
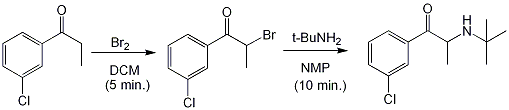
Chemicals
m-chloropropiophenone,
dichloromethane,
bromine,
1-Methyl-2-pyrrolidinone,
t-butylamine,
diethylether,
concentrated hydrochloric acid,
isopropanol,
MgSO4 (anhydrous)
dichloromethane,
bromine,
1-Methyl-2-pyrrolidinone,
t-butylamine,
diethylether,
concentrated hydrochloric acid,
isopropanol,
MgSO4 (anhydrous)
Procedure
To a stirred solution of m-chloropropiophenone (1 g, 5.9 mmol) and dichloromethane (5.0 mL) there was added, portionwise, 6.0 mL of a 1.0 M solution of bromine in dichloromethane. The solvent was removed under vacuum shortly after the addition was compleated and replaced by 1-Methyl-2-pyrrolidinone (5 mL) followed by t-butylamine (5 mL, 47.5 mmol). The flask was heated with stirring to 60°C for 10 minutes. The obtained red solution was treated with water (25 mL) and extracted with ether (3 x 25 mL), the combined ether fractions were washed with water (5 x 25 mL) and dried over anhydrous MgSO4. The resulting yellow solution was transferred to a beaker chilled on ice and slowly with stirring treated with a 20:100 v/v mixture of conc. HCl in isopropanol until the contents of the beaker were acid to pH paper. (+/-)-2-(tert-butylamino)-1-(3-chlorophenyl)propan-1-one hydrochloride was collected with suction, washed with ether and allowed to dry, yielding 1 g (70%) of pure white crystals.
Author Comments
(+/-)-2-(tert-butylamino)-1-(3-chlorophenyl)propan-1-one hydrochloride and some of it's analogues have previously been prepared by bromination of an arylketone with either dioxane dibromide[1] or bromine in methanol[2], subsequent treatment with desired amine gave aminoketones.
However, the synthesis of the title compound can effectively be completed in less than an hour, and in high yield, by using NMP as solvent during the substitution step.
This reaction has been conducted by a number of undergraduates only on a 6 mmol sacale, the yields of the final product ranged from 70-80%. This protocol has never failed and it has not been applied to a different system to my knowledge.
It is advisable to use 1 M concentration of bromine in DCM instead of pure bromine and of course to conduct the bromination in a fume-hood.
The sequence is easy and reliable and the yield is high. It is not recommended to isolate the intermediate haloketone owing to its potent lachrymatory properties. The amine product is a dopamine and norepinephrine reuptake inhibitor.
Data
1H NMR 300 MHz (CDCl3): 7.98 (1H, t), 7.86 (1H, dt), 7.7 (1H, dt), 7.55 (1H, t), 4.85 (1H, q), 1.93 (3H, d), 1.51 (9H, s)
TLC with EtOAc as eluent: Rf(final product as hydrochloride): 0, Rf(m-chloropropiophenone): 0.95
Other References
[1] Pasaribu, S. J.; Williams, L. R. Aust. J. Chem. 1973, 26, 1327.
[2] Mehta, N. B.; Musso D. L. J. Pharm. Sci. 1986, 75, 410.
Keywords
Comments
In the second step are you sure you have not got any imine?
By Muneer Ahamed on November 9, 2007
great! but is it possible to use the procedure till halogention step ?
By santosh lahore on November 12, 2007
NMR analysis showed no presence of imine. Alpha-bromo ketones don't follow condensation pathway when treated with amine nucleophiles as described above.
Yes, it is of course possible to stop at the bromoketone stage.
This experiment was a part of the undergraduate laboratory course which I took at the time the above report was posted. Recently I found (by chance) the paper from which the method originated; Journal of Chemical Education, vol. 77, Issue 11, p.1479.
By Vedran Hasimbegovic on December 8, 2007
NMR analysis showed no presence of imine. Alpha-bromo ketones don't follow condensation pathway when treated with amine nucleophiles as described above.
Yes, it is of course possible to stop at the bromoketone stage.
This experiment was a part of the undergraduate laboratory course which I took at the time the above report was posted. Recently I found (by chance) the paper from which the method originated; Journal of Chemical Education, vol. 77, Issue 11, p.1479.
By Vedran Hasimbegovic on December 8, 2007
I forgot to add the publication year (2000) of the paper, abstract can be found with this link: http://adsabs.harvard.edu/abs/2000JChEd..77.1479P
This additional info can be simply added to my previous comment.
Thanks!
By Vedran Hasimbegovic on December 8, 2007
I forgot to add the publication year (2000) of the paper, abstract can be found with this link: http://adsabs.harvard.edu/abs/2000JChEd..77.1479P
This additional info can be simply added to my previous comment.
Thanks!
By Vedran Hasimbegovic on December 8, 2007