Phosphonylation of an Alkyl Bromide
SyntheticPage 226
DOI:
Submitted: May 19, 2005, published: May 19, 2005
Authors
C. Michele Davis-McGibony (mdavis@georgiasouthern.edu)
A contribution from
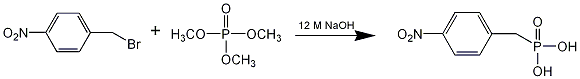
Chemicals
4-nitrobenzyl bromide (Aldrich)
trimethyl phosphite (Aldrich)
NaOH (Fisher)
Diethyl Ether (Fisher)
trimethyl phosphite (Aldrich)
NaOH (Fisher)
Diethyl Ether (Fisher)
Procedure
4-Nitrobenzyl bromide (0.94 g, 4.3 mmole) was added to an excess of trimethyl phosphite (10.52 g, 89 mmol). While stirring, NaOH (1 mL of 12.5M) was added dropwise to the solution. After stirring for 24 hours at room temperature, the solution was filtered to yield 1,4-nitrobenzylphosphonic acid as a yellow-beige solid, which was recrystallized in ethyl ether to yield glassy crystals (0.54 g; 70.5%).
Author Comments
This reaction is a quick one-pot synthesis of 1,4-nitrobenzylphosphonic acid. May be reliable with other compounds. Be careful of trimethyl phosphate. It emits a distinctive, pungent, pyridine-like odor and can cause eye or skin irritation upon contact. Wear gloves and use proper ventilation. As soon as the NaOH was added, a color change occurred (clear solution to a red-rust solution) and some solid precipitated immediately. After 24 hours, the precipitate was a yellow-beige color, suspended in solution.
Data
mp = 215°C; 1H NMR (250 MHz, DMSO-d6) : 3.5 (d, 2H), 5.4 (s, 1H), 7.5 (m, 2H), 8.25 (m, 2H)
Lead Reference
Bennett, R. R. and Davis, C. Michele, 54th Southeastern Regional Meeting of the American Chemical Society, Charleston, SC, November 13-16, 2002, Abstract #781.
Keywords
Comments
The chemical used in the reaction is trimethyl phosphite but the reagent in the picture is trimethyl phosphate.
By Antti Siiskonen on January 8, 2006
This is arbuzov reaction,but the moleculer‘s structure of trimethyl phosphite is wrong .
By zhenhua zhao on November 23, 2007