Darzens condensation
SyntheticPage 225
DOI:
Submitted: May 10, 2005, published: May 11, 2005
Authors
Kirtikumar B. Jadhav (kirtikumarjadhav@gmail.com)
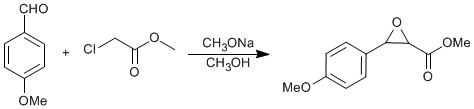
Chemicals
p-Methoxy benzaldehyde,
Methyl chloroacetate,
Methanol [distilled from Na],
Sodium metal
Methyl chloroacetate,
Methanol [distilled from Na],
Sodium metal
Procedure
To a solution of 5.1g (0.22 gram-atoms) of Na in 90mL of anhydrous MeOH, chilled to -10ºC in an ice bath, a solution of 20g (0.15 moles) of 4-Methoxy benzaldehyde and 23.9g (0.22 moles) of Methyl chloroacetate was added dropwise over a period of 3h, during which time the reaction mixture was vigorously stirred and finally the whole became a white paste. After the addition was completed, the mixture was stirred at -5ºC for 2h and then at room temperature for 3h. The mixture was poured into ice-water (350mL) containing AcOH (2mL); the precipitated white solid was filtered, washed with cold water and dried in a dessicator. The crude glycidate weighed 23g (75%). It was recrystallized from MeOH to give pure Methyl glycidate.
Author Comments
It’s imperative to maintain inert (N2) atmosphere throughout the course of reaction. The glycidic ester is an important key intermediate in the synthesis of Diltiazem – a Ca2+ Channel blocker. The diastereomeric mixture obtained can further be purified by enzymatic methods in order to gain access to diltiazem.
Data
Melting range: 68-69ºC.
1H NMR (300MHz, CDCl3): 7.21 (2H, d, J = 8.6Hz), 6.89 (2H, d, J = 8.6Hz), 4.05 (1H, m*), 3.51 (1H, m*), 3.82 (3H, s), 3.80 (3H, s).
[* = as the reaction yields inseparable mixture of cis- and trans-glycidic ester, well resolved doublets for epoxide CH protons were not obtained].
APCI MS: 208.9 (M+1).
FT-IR (KBr, thin plate, ): 3020.2 cm-1 (w, sp2 C-H str), 2954.4 cm-1 (w, asymm. sp3 C-H str), 2837.7 cm-1 (w, symm. sp3 C-H str), 1728.3 cm-1 (vs, ester C=O str), 1613.8 - 1437 cm-1 (m, aro. skeletal C=C vibrations), 1311.7 cm-1 (m), 1294.0 cm-1 (m, symm str of epoxide), 1251.5 cm-1 (s, asymm. Ar-O-C str), 1179.0 cm-1 (w), 1029.4 cm-1 (s, symm Ar-O-C str), 856.1 cm-1 (w), 840.4 cm-1 (m, para subst. oop bend), 800.3 cm-1 (w), 774.2 cm-1( w).
Lead Reference
1. Ban, Y.; Oishi, T. Chem. Pharm. Bull., 1958, 6, 574-576.
2. Crotti, P.; Ferretti, M; Macchia, F. J. Org. Chem. 1986, 51, 2759-2766.
Other References
1. Bachelor, F.; Bansal, R. ‘The Darzens Glycidic Ester Condensation’ J. Org. Chem. 1969, 34, 3600-3604.
2. Tung, C.; Speziale, A.; Frazier, H. ‘The darzens condensation. II. Reaction of chloroacetamides with aromatic aldehydes.’ J. Org. Chem. 1963, 28, 1514-1521.
Keywords
Comments
the yield is lower.
By dengdecai on March 22, 2006
Why inert (N2) atmosphere should needed throughout the course of reaction?
By yongjuna jia on November 11, 2006
(N2) atmosphere should not needed.
By dengdecai on April 29, 2007