Synthesis of E,E-dibenzylidene acetone (dba) and aryl substituted derivatives
SyntheticPage 221
DOI:
Submitted: December 8, 2004, published: December 17, 2004
Authors
Dr. Ian J. S. Fairlamb (ijsf1@york.ac.uk)
Chemicals
Aldehydes were purchased from Lancaster or Aldrich. Acetone and ethanol were purchased from Merck. We used all materials as recieved - no special purification was employed
Procedure
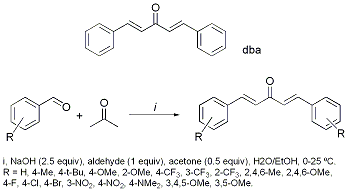
To a 500ml conical flask equipped with a magnetic stirrer was added a solution of sodium hydroxide (4.72 g, 0.118 mol, 2.5 eqv.) in water (47 mL). Ethanol (33 mL) was added with stirring and the solution was cooled by an ice-water bath. To the resultant solution, benzaldehyde (5 g, 0.047 mol, 1 eqv.) and analytical grade acetone (1.36 g, 0.023 mol, 0.5 eqv.) were added slowly over 15 minutes. On complete addition, the mixture was stirred for one hour at room temperature. A yellow solid appeared slowly, and after a further hour it was filtered in vacuo and then washed with ether (3 x 50 mL). The solid material could be purified by flash chromatography using petroleum ether 40-60°C/ethyl acetate (4/1, v/v) to afford dibenzylidene acetone as a yellow solid (3.98 g, 74 %) or recrystallised from CH2Cl2.
Author Comments
The alpha,beta-unsaturated carbonyl compound, E,E-dibenzylidene acetone (dba or 1,5-bis-penta-1E,4E-dien-3-one), is readily synthesised by simple base-mediated aldol condensation of two equivalents of benzaldehyde with acetone in the presence of sodium hydroxide in a water/ethanol solvent medium at room temperature (ref. 1). A literature survey reveals that other methods have been described. For example, a titanium tetralkoxide-induced aldol condensation has been reported for the synthesis of dba and derivatives (containing alkenic substitution) (ref. 2). Although this reaction occurs under neutral conditions, it requires up to 4 days to reach completion. An alternative procedure employs a partially dehydrated barium hydroxide catalyst (C-200, heterogeneous) for aldol condensation, under reflux with ethanol, to give dba in yields >95% in 1 h, although no substituted dba derivatives were reported (ref. 3). An indium trichloride mediated reaction of two equivalents of benzaldehyde with acetone provides dba in 88% yield, but requires 16 h reaction time at 110 degrees celcius in a sealed tube (ref. 4). A microwave assisted variant, mediated by KF-Al2O3, has further been reported from dba (ref. 5). We have established that the best practical method is the classic way using the simple base-mediated aldol condensation reaction. The KEY point is in the due care and attention required in the purification of these derivatives. Compounds were purified according to the following key: A: The product precipitates. Filtered and washed with ether. Filtrate concentrated in vacuo, then recrystallised from CH2Cl2. B: Extracted into CH2Cl2, washed with water, then sat. aq. NaCl, dried (MgSO4) and concentrated in vacuo. Purified by flash chromatography using ethyl acetate/hexane mixtures. C: As for B, but then recrystallization with THF. D: As for A, but taken up in toluene (aldehyde insoluble in this). Filter, concentrate filtrate and recrystallize from hexane. R = 4-CH3, (Method A); R = 4-t-Bu, (Method C); R = 4-OMe, (Method A);R = 2-OMe, (Method A); R = 4-CF3, (Method A); R = 3-CF3, (Method A); R = 2-CF3, (Method A); R = 2,4,6-Me, (Method A); R = 2,4,6-OMe, (Method B); R = 4-F, (Method A); R = 4-Cl, (Method A); R = 4-Br, (Method D); R = 3-NO2, (Method C); R = 4-NO2, (Method C); R = 4-NMe2, (Method B); R = 3,4,5-OMe, (Method B); R = 3,5-OMe, (Method B). The isolated yields in the vast majority of cases are very good, on a relatively large scale (5-50 g). If you are wondering what to do with all these dba derivatives, see ref. 6. A complete (non refereed paper has been prepared by our group and available at: http://www-users.york.ac.uk/%7Eijsf1/dba-derivatives.pdf).
Data
selected data: 1,5-Bis-penta-1E,4E-dien-3-one Mp 120-121 oC; 1H 7.75 (d, 2H, 3J=16.0, H1), 7.61 (4H, m, H2', H6'), 7.42 (6H, m, H3', H5', H4'), 7.09 (2H, d, 3J=16.0, H2); 13C 189.0, 143.4, 134.9, 130.6, 129.0, 128.5, 125.5.IR (CH2Cl2, cm-1) 1657m, 1651m (C=O), 1627vs (C=C), 1591w (C=C aromatic), 1574w (C=C aromatic), 983m (CH trans); UV (THF nm) 233 (pi-pi*), 321 (n-pi*); LRMS (EI) m/z 234 (M+, 100), 205 (10), 131 (55), 103 (75), 91 (30), 77 (75), 51 (40); HRMS (EI) m/z C17H14O calculated mass of 234.1102, found 234.1103. 1,5-Bis-(3',5'-dimethoxyphenyl)penta-1E,4E-dien-3-one Mp 132-133 oC; 1H 7.62 (2H, d, 3J=8.2, H4'), 7.01 (2H, d, 3J=15.7, H1), 6.71 (4H, t, 3J=7.6, 7.9, H6'), 6.48 (2H, d, 3J=15.7, H2), 3.70 (12H, s); 13C 188.7, 160.9, 143.2, 136.5, 125.5, 106.1, 102.7, 55.3. IR (CH2Cl2, cm-1) 1652m (C=O), 1621vs (C=C), 1596w (C=C aromatic), 1572w (C=C aromatic), 989m (CH trans); LRMS (EI) m/z 354 (M+, 100), 203 (10); HRMS (EI) m/z C21H22O5 calcd. mass of 354.1467, found 354.1485. 1,5-Bis-(3',4',5'-trimethoxyphenyl)penta-1E,4E-dien-3-one Mp 155-156 oC; 1H 7.55 (1H, d, 3J=15.8, H1), 6.94 (1H, d, 3J=15.8, H2), 6.77 (2H, s, 3J=7.8, H2', H6'); 3.78 (12H, s, 4xOCH3(meta)), 3.72 (s, 2xOCH3(para)); 13C 188.5, 153.9, 143.2, 140.7, 130.6, 125.2, 105.8, 60.9, 56.4. IR (CH2Cl2, cm-1): 1648m (C=O), 1617vs (C=C), 1583w (C=C aromatic), 1504w (C=C aromatic), 1000m (CH trans); LRMS (EI) m/z: 414 (M+, 45), 399 (15), 383 (15), 181 (100); HRMS (EI) m/z: C23H26O7 calcd. mass of 414.1678, C23H26O7 requires 414.1679. 1,5-Bis-(2',4',6'-trimethoxyphenyl)penta-1E,4E-dien-3-one Mp 202-204 oC; 1H 8.01 (2H, d, 3J=16.1, H1), 7.37 (2H, d, 3J=16.1, H2), 6.12 (4H, s, 3J=8.3, H3Œ, H5Œ); 3.87 (12H, s, 4xOCH3(ortho)), 3.80 (6H, s, 2xOCH3(para)); 13C 189.7, 160.8, 159.4, 130.7, 124.4, 104.2, 88.5, 53.5, 53.2. IR (CH2Cl2, cm-1): 1620m (C=O), 1598vs (C=C), 1572w (C=C aromatic), 997m (CH trans); LRMS (EI) m/z: 414 (M+, 15), 399 (10), 383 (100); HRMS (EI) m/z: C23H26O7 calcd. mass of 414.1678, C23H26O7 requires 414.1687. 1,5-Bis-(3'-nitroophenyl)penta-1E,4E-dien-3-one Mp 147-148 oC; 1H 8.47 (2H, t, 4J=1.8, H2'), 8.21 (2H, ddd, 3J=8.2, 4J=2.2, 0.9, H4'), 7.91 (2H, dd, 3J=7.9, 4J=0.9, H6'), 7.73 (2H, d, 3J=15.9, H1), 7.59 (2H, t, 3J=7.9, H5'), 7.17 (2H, d, 3J=5.9, H2); 13C 206.8, 175.7, 141.1, 134.5, 130.5, 129.0, 127.9, 125.1, 122.9. IR (KBr, cm-1) 1677m (C=O), 1633vs (C=C), 1600w (C=C aromatic), 1573w (C=C aromatic), 1510s (NO2 asym.), 1350s (NO2 sym.), 992m (CH trans); LRMS (EI) m/z 324 (M+, 48), 307 (47), 202 (19), 176 (67), 102 (100); HRMS (EI) m/z C17H11O5N2 calcd. mass of 324.0732, found 324.0744.
Lead Reference
ref 1. Conard, C. R.; Dolliver, M. A. Org. Synth. 1943, 2, 167.
Other References
ref 2. Mahrwald, R.; Schick, H. Synthesis 1990, 592;
ref 3. Sinisterra, J. V.; Garcia-Raso, A.; Cabello, J. A.; Marinas, J. M. Synthesis 1984, 502;
ref 4. Deng, G.; Ren, T. Synth. Commun. 2003, 33, 2995;
ref 5. Yadav, J. S.; Sub Reddy, B. V.; Nagaraju, A.; Sarma, J. A. R. P. Synth. Commun. 2002, 32, 893;
ref 6. Fairlamb, I. J. S.; Kapdi, A. R.; Lee, A. F. Org. Lett. 2004, 6, 4435.
Keywords
aldol, alkenes, base-catalysed, ketones
Comments
I think that somemething is missing in the procedure. where is the benzaldehyde?
By Mauricio Rosa on June 27, 2005
This should be OK now.
By Peter Scott on June 28, 2005
http://www-users.york.ac.uk/%7Eijsf1/dba-derivatives.pdf
This is not available.
By wu huajiang on December 6, 2007
http://www-users.york.ac.uk/%7Eijsf1/dba-derivatives.pdf
This PDF data is not available a year later. Perhaps causing it to be available would be helpful ?
By Mark Bertinetti on November 4, 2008