Amination of a glyoxalyl chloride
SyntheticPage 220
DOI:
Submitted: August 10, 2004, published: August 10, 2004
Authors
Christine R. Whitlock (cwhitlock@georgiasouthern.edu)
A contribution from
Chemicals
indole (Aldrich)
diethyl ether (Fisher)
oxalyl chloride (Aldrich)
tris(2-aminoethyl)amine (Aldrich)
triethylamine (Aldrich)
THF (Aldrich)
methanol (Fisher)
diethyl ether (Fisher)
oxalyl chloride (Aldrich)
tris(2-aminoethyl)amine (Aldrich)
triethylamine (Aldrich)
THF (Aldrich)
methanol (Fisher)
Procedure
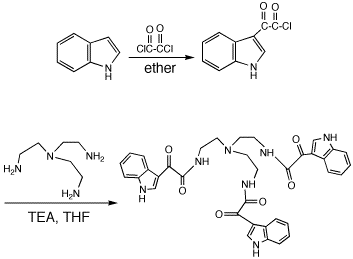
To indole (10.07 g, 86.1 mmol) in ether (200 mL) was added dropwise oxalyl choride (14.6 g, 114.6 mmol) over 15 mins. After stirring for 30 min, the solution was filtered to yield indole-3-glyoxalyl chloride as a yellow solid, which was rapidly dissolved in THF (50 mL). To the indole-3-glyoxalyl chloride in THF at 0°C was added a solution of tris(2-aminoethyl)amine (6.14 g, 42.0 mmol) and triethylamine (42.3 mmol) in THF (10 mL). After stirring at 0°C for 1 h, the solution was filtered and the filtrate was evaporated to yield tris(2-[indole-3-glyoxylamido]ethyl)amine as a beige solid, which was recrystallized in MeOH to yield glassy crystals.
Author Comments
The reaction is an efficient way to prepare tris(2-[indole-3-glyoxylamido]ethyl)amine. The glyoxalyl choride intermediate is unstable, thus cold temperatures are necessary. Could be reliable with other acyl chlorides.
Data
Yield 5.93 g (31.4%); mp > 250°C. 1H NMR (250 MHz, DMSO-d6) _8.75 (s, 3H), 8.65 (t, J=5.29 Hz, 3H), 8.14 (d, J=6.9 Hz, 3H), 7.47 (d, J=7.1 Hz, 3H), 7.22 (t, J=7.0 Hz, 3H), 7.16 (t, J=7.0 Hz, 3H), 3.31 (m, 6H, Ha), 2.71 (bt, 6H, Hb); MS (M+1)+ = 660 for C36H33N7O6
Lead Reference
Carpenter, R. A.; Farley, A. R.; Cox, J. R.; Dobson, A. J.; Whitlock, C. R. J. Undergrad. Chem. Res. 2004, 1, 114.
Keywords
Comments
Here we are using TEA.Instead of TEA can we use Ammonia/urea.Is it possibble
Thanking you sir
By UDAYA RAO on June 25, 2006
I want to discuss the remarks from UDAYA RAO, It is not possible to use ammonia in this reaction, AS The author is reporting the condensation with tris(2-aminoethyl)amine. TEA, being tertiary amine can't react with the indole-3-glyoxalyl chloride rather Ammonia and urea do react, resulting in the formation of different products other than the required one.
By Matloob Ahmad on January 11, 2008