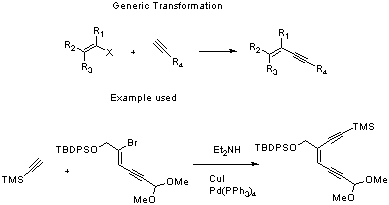
Sonogashira Coupling of alkynes to vinyl halides
SyntheticPage 21
DOI:
Submitted: July 2, 2001, published: July 2, 2001
Authors
zac etheridge (zac.etheridge@orange.net)
A contribution from
Chemicals
Palladium tetrakis(triphenylphosphine) (1-2mol%, 100mg)
Diethylamine (distilled, 2-2.5mL/mmol alkyne, 10mL)
Copper iodide (washed with dry THF and dried in vacuo, 5-6mol%, 41mg)
vinyl halide (1 equiv, 4.36mmol, 2.06g)
alkyne (1.2 equiv, 0.72mL, 5.23mmol)
Diethylamine (distilled, 2-2.5mL/mmol alkyne, 10mL)
Copper iodide (washed with dry THF and dried in vacuo, 5-6mol%, 41mg)
vinyl halide (1 equiv, 4.36mmol, 2.06g)
alkyne (1.2 equiv, 0.72mL, 5.23mmol)
Procedure
The palladium tetrakis(triphenylphosphine), diethylamine and vinyl halide are stirred together at room temperature for 15 minutes. The copper iodide is then added, followed by the alkyne. The solution is heated for 3-5 hours (TLC) at reflux, during which time it will usually go brown. The reaction is quenched with ammonium chloride, extracted with ether, washed with brine, dried over magnesium sulphate, filtered, concentrated and purified by column chromatography.
Author Comments
This reaction is also apparently possible using commercially available PdCl2(PPh3)2 but yields tend to be either lower or non-existant compared to palladium tetrakis(triphenylphosphine)(a synthetic page for the synthesis of this is also available). The reaction should be carried out under anhydrous conditions due to the sensitivity of the catalyst to moisture, and care should be taken when analysing the reaction by TLC as the product and starting material often run very close together. Yields of 80-90% are generally possible.
Data
1H NMR (300MHz, CDCl3) 7.48 (4H, m), 7.24 (6H, m), 6.42 (1H, dd, J=2.0, 1.5), 5.17 (1H, d J=1.5), 4.16 (2H, d, J=1.9), 3.27 (6H, s), 0.90 (9H, s), 0.00 (9H, s).
Lead Reference
Caddick S., Delisser V. Tetrahedron Lett. 1997 p2355
Other References
Brandsma L., Vasilevsky S.F., Verkruijsse H.D. Applications of Transition Metal Catalysts in Organic Synthesis, p179-225.
Sonogashira K. Tetrahedron Lett. 1975 p4476