DCC mediated coupling of oximes and carboxylic acids.
SyntheticPage 19
DOI:
Submitted: June 28, 2001, published: June 28, 2001
Authors
Andrew McCarroll (andrew.mccarroll@pharminox.com)
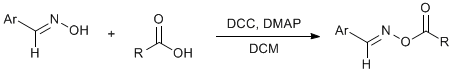
Chemicals
"Carboxylic acid, prepared or used as received, 1 equiv"
Aryl aldoxime (prepared from aryl aldehyde, 1 equiv)
DCC (1 equiv, as received)
DMAP (~ 0.01 g/ mmol, as received)
Dichloromethane (undistilled)
Aryl aldoxime (prepared from aryl aldehyde, 1 equiv)
DCC (1 equiv, as received)
DMAP (~ 0.01 g/ mmol, as received)
Dichloromethane (undistilled)
Procedure
The reagents are mixed together at 0°C (DCC added last), and stirred for six hours at this temperature. (It is not crucial to keep temp at 0°C constantly - reaction can be left to stir overnight. However best results are obtained if mixture is cooled prior to filtering: No DCU impurity is obtained.) The mixture is filtered to remove the DCU by-product, and the mixture is concentrated. Best results are obtained when this is done slowly without heating - crystallisation is easier. Purification is by recrystallisation (DCM/hexane or toluene/hexane) or column chromatography (PE/EtOAc or PE/DCM).
Author Comments
The reaction works well for the synthesis of derivatives of benzaldoximes, pentafluorobenzaldoximes, 4-nitrobenzaldoximes, 2,4-dimethoxybenzaldoximes, and 2,4,6-trimethoxybenzaldoximes, as well as benzophenone oximes. The method has not been investigated for the synthesis of anti- aldoximes or alkylaldoximes. The method has only been found to fail when the product oxime esters are unstable (O-trihalomethyl aldoximes). Yields are good to excellent. Benzaldoxime derivatives are rarely crystalline, and chromatography is required. 2,4 dimethoxybenzaldoxime and pentafluorobenzaldoxime derivatives can sometimes be obtained in crystalline form, while p-nitro and 2,4,6-trimethoxybenzaldoxime derivatives are usually crystalline. Recrystallisation can usually be performed easily and efficiently in either of the solvent mixtures. Use of neat toluene or DCM results in loss of product due to the low volume of solvent required. The presence of DMAP may (in our hands) or may not (see Rigny and Samne) be crucial.
Data
Example data for O-trimethylacetyl benzaldoxime: 1.32 (9H, s), 7.44–7.50 (3H, m), 7.75 (2H, d, J = 6.2 Hz), 8.38 (1H, s).
Lead Reference
A. J. McCarroll and J. C. Walton, J. Chem. Soc., Perkin Trans. 2, 2000, 2399.
Other References
M. Hasebe and T. Tsuchiya, Tetrahedron Lett., 1986, 27, 3239. R. Rigny and S. Samne, Compt. Rendu Acad. Sc. Paris C, 1968, 266, 1303. The instability of some oxime esters was previously reported: M. Benger and O. Brady, J. Chem. Soc., 1950, 1221. For a review of carbodiimide chemistry, see A. Williams and I. Ibrahim, Chem. Rev., 1981, 81, 589.
Keywords
amino acids, carbodiimide, carboxylic acids, coupling, DCC, DCU, DMAP, O-acyl oximes, oxime esters