Oxidative cleavage of an alkene with catalytic ruthenium tetroxide
SyntheticPage 186
DOI:
Submitted: March 11, 2002, published: March 12, 2002
Authors
Meriel Kimberley (meriel.kimberley@bristol.ac.uk)
A contribution from
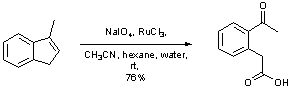
Chemicals
3-methyl-indene,
sodium periodate (Aldrich),
ruthenium trichloride hydrate (Aldrich),
acetonitrile (HPLC grade, Aldrich),
hexane
water
sodium periodate (Aldrich),
ruthenium trichloride hydrate (Aldrich),
acetonitrile (HPLC grade, Aldrich),
hexane
water
Procedure
To a solution of 3-methyl-indene (497 mg, 3.8 mmol) in acetonitrile (7 ml), hexane (7 ml) and water (10 ml) was added sodium periodate (3.35 g, 15.7 mmol) followed by ruthenium trichloride (17 mg, 0.08 mmol, 2.2 mol%). The entire mixture was then stirred at room temperature for 2 hours until the reaction was complete (TLC). Dichloromethane was then added (30 ml), the phases separated and the upper phase was extracted with dichloromethane (3 x 30 ml). The combined organic extracts were dried (MgSO4) and concentrated in vacuo. Diethyl ether was added to the resulting residue (to precipitate any remaining inorganic species). The resulting solution was filtered through a pad of celite and concentrated to afford the crude keto-acid which could be used without further purification. Pure compound can be obtained by recrystallisation from ethyl acetate.
Author Comments
Care should be taken to ensure vigorous stirring after addition of ruthenium trichloride as the reaction solution can get very warm.
Data
1H (CDCl3): 7.84 (1H, d, J 7.7 Hz, CH), 7.51 (1H, t, J 7.7 Hz, CH), 7.42 (1H, t, J 8 Hz), 7.35 (1H, d, J 8 Hz, CH), 3.91 (2H, s, CH2) and 2.65 (3H, s, CH3).
Lead Reference
P. H. Carlsen, T. Katsuki, V. S. Martin, K. B. Sharpless, J.Org. Chem., 1981, 46, 19, 3936-3938.
Other References
M. Shindo, Y. Sato, K. Shishido, J. Org. Chem., 2001, 66, 23, 7818-7824.