Dieckmann Condensation reaction
SyntheticPage 176
DOI:
Submitted: November 28, 2001, published: November 28, 2001
Authors
lisa frost (lisa.frost@evotec.com)
A contribution from
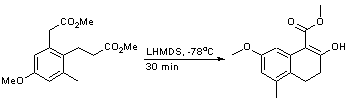
Chemicals
3-(4-methoxy-2-methoxycarbonylmethyl-6-tolyl)-propionic acid methyl ester SP:XX
Lithium bis(trimethylsilyl)amide (1M in Hexane) Aldrich
THF (distilled from benzophenone ketyl)
Lithium bis(trimethylsilyl)amide (1M in Hexane) Aldrich
THF (distilled from benzophenone ketyl)
Procedure
To a solution of 3-(4-methoxy-2-methoxycarbonylmethyl-6-tolyl)-propionic acid methyl ester (6.32 g, 23 mmol, 1 equiv) in THF (170 mL)cooled to -78 oCwas added LHMDS (27 mL of a 1M solution in hexanes, 27 mmol, 1.2 equiv) dropwise. The reaction was allowed to stir at this temperature for 30 minutes producing a red solution. TLC analysis [9:1; Pet:EtOAc] identified complete product formation. The reaction was quenched with NH4Cl solution (40 mL) and extracted with ethyl acetate (2 x 100 mL), dried (MgSO4), filtered and concentrated in vacuo. Purification was achieved by chromatography on silica gel with Pet:EtOAc (9:1 v:v) to yield the title compound as a white solid (2.22 g, 40%).
Author Comments
Although this reaction seems poor yielding, the conditions have been optimised for this system and this is about the best yield obtained. The reaction yield is greatly reduced if the length of reaction is increased or the temperature is altered. Further, no SM is reovered, presumably the reaction yield is low due to decomposition of the SM under the harsh base conditions.
Data
1H nmr (300 MHz, CDCl3) 7.18 (1H, d, J 2.4 Hz), 6.56 (1H, d, J = 2.4 Hz), 3.92 (3H, s), 3.82 (3H, s), 2.75-2.65 (2H, m), 2.55-2.45 (2H, m), 2.30 (3H, s).
Lead Reference
Rucker, Mark; Brueckner, Reinhard; Synlett.; 1997; 1187-1189
Keywords
Comments
For formation of 3-(4-methoxy-2-methoxycarbonylmethyl-6-tolyl)-propionic acid methyl ester see [[174|Page 174]]
By lisa frost on November 28, 2001