Formation of a methyl ester under acid conditions
SyntheticPage 174
DOI:
Submitted: November 27, 2001, published: November 28, 2001
Authors
lisa frost (lisa.frost@evotec.com)
A contribution from
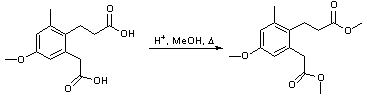
Chemicals
3-(2-carboxymethyl-4-methoxy-6-tolyl)-propionic acid SP:XX
Methanol (GPR)
H2SO4
Methanol (GPR)
H2SO4
Procedure
To a solution of the diacid (6.56 g, 26 mmol, 1 equiv) in methanol (200 mL) was added a catalytic amount of conc. H2SO4 (0.07 mL, 1.3 mmol, 5 mol%). The mixture was set to reflux for 6 h after which it was allowed to cool to room temperature. The methanol was removed in vacuo and the resulting oil washed with sat. NaHCO3 solution (20 mL). The organics were extracted into ethyl acetate (3 x 50 mL), dried (MgSO4), filtered and concentrated in vacuo leaving a brown oil which was seen to be of sufficient purity for use in further reactions. (6.32 g, 87%).
Author Comments
This reaction is simple to carry out and is high yielding and also yields high purity material. It has been carried out on various scales, all of which have been high yielding.
Data
1H nmr (300 MHz, CDCl3) 6.69 (1H, d, J = 2.5 Hz), 6.66 (1H, d, J = 2.5 Hz), 3.78 (3H, s), 3.72 (3H, s), 3.71 (3H, s), 3.67 (2H, s), 2.94-2.88 (2H, m), 2.45-2.37 (2H, m), 2.34 (3H, s)
Lead Reference
Rucker, Mark; Brueckner, Reinhard; Synlett.; 1997; 1187-1189.
Keywords
Esterification, Naphthoic acid, 174