Nucleophilic displacement of a benzylic bromide using sodium cyanide
SyntheticPage 172
DOI:
Submitted: November 26, 2001, published: November 26, 2001
Authors
lisa frost (lisa.frost@evotec.com)
A contribution from
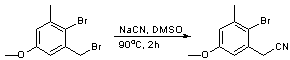
Chemicals
2-bromo-1-bromomethyl-5-methoxy-3-methyl benzene ([[156|Page 156]])
Sodium cyanide (Lancaster)
DMSO (Avocado)
Sodium cyanide (Lancaster)
DMSO (Avocado)
Procedure
To a solution of 2-bromo-1-bromomethyl-5-methoxy-3-methyl benzene (24.7g, 0.084 mol, 1 equiv) in DMSO (1.25 mL/mmol; 105 mL) was added a solution of sodium cyanide (7.4 g, 0.151 mol, 1.8 equiv) in DMSO (40 mL) by aid of a dropping funnel. The resulting golden solution was heated to 90 oC for 2 h and allowed to cool to room temperature. The resulting brown solution was poured into 1.5 L of ice water to give a fawn cloudy solution. This was extracted with ether (2.5 L in total) to give a brown solution. The combined organic layers were dried over sodium sulfate and concentrated in vacuo to yield a brown solid which was seen to be of sufficient purity for use in further stages. (17.6 g, 87%)
Author Comments
This reaction has been carried out numerous times within the lab on both large and small scales. We have found that the largest scale possible due to work up considerations is the scale reported above. The addition of the sodium cyanide can sometimes cause problems due to the low solubility in DMSO. This problem is easily overcome by simply adding the NaCN portionwise to a more dilute solution of the bromide. A further practical consideration is the aqueous extraction, which sometimes causes problems due to the DMSO preventing separation of the two layers. This can be overcome by the addition of a small amount of salt to the aqueous layer. NB All glasswear must be cleaned by submersion in bleach for 24 h after the reaction. Local safety rules should be consulted before working with cyanides (eg breathing apparatus, antidote etc).
Data
1H nmr (300MHz, CDCl3) 2.41 (2H, s), 3.82 (3H, s), 3.84 (3H, s), 6.79 (1H, d, J = 2.7Hz), 6.93 (1H, d, J =2.7Hz).
Lead Reference
Rucker, Mark; Brueckner, Reinhard; Synlett.; 1997; 1187-1189.
Other References
Goerth, Felix Christian; Rucker, Mark; Eckhardt, Matthias; Brueckner, Reinhard; Eur.J.Org.Chem.; 14; 2000; 2605 - 2612.