Preparation of tri-o-tolylphosphine: A useful ligand for high temperature Heck coupling reactions
SyntheticPage 170
DOI:
Submitted: November 21, 2001, published: November 22, 2001
Authors
lisa frost (lisa.frost@evotec.com)
A contribution from
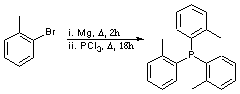
Chemicals
2-Bromotoluene (Avocado)
Magnesium turnings (Avocado)
Phosphorus trichloride (BDH)
THF (distilled from benzophenone ketyl)
Magnesium turnings (Avocado)
Phosphorus trichloride (BDH)
THF (distilled from benzophenone ketyl)
Procedure
To a solution of magnesuim turnings (3.11 g, 128 mmol, 3.5 equiv) in THF (50 mL) was added a small amount of 2-bromotoluene (1 mL) and 1 iodine crystal. The reaction vessel was heated until initiation occurred, where upon a solution of the remaining 2-bromotoluene (20 g, 117 mmol, 3.2 equiv in total) in THF (100 mL) was added. The reaction was set to reflux for 2 hours (colour change to black solution), after which it was cooled to 0 oC and a solution of phosphorus trichloride (5.01 g, 36.5 mmol, 1 equiv) in THF (30 mL) was added dropwise. Upon addition completion the reaction was set to reflux for 18 hours. The reaction was allowed to cool to room temperature, whereupon NH4Cl solution was added with care. The resulting solution was extracted with ether (3 x 100 mL), dried over MgSO4, filtered and concentrated in vacuo. The resulting white solid was recrystalised from ethanol to yield the title compound as a white solid. (6.8 g, 62 %)
Author Comments
Although this compound is commercially available it is relatively expensive seeing as it is so easy to prepare. The Grignard reaction is a little temperamental and may need a little encouragement to initiate. A word of warning; don't add all of the bromotoluene in one portion as this will swamp the magnesium and the reaction will never work. Further, if you find that the iodine is not enough to initiate the reaction, we have found that a microspatula of mercuric chloride may help for this and other Grignard reactions. This reaction has been carried out on various scales and has shown to yield about 60% of the required product in all cases.
Data
m.pt. 125-127 oC
31P nmr (120MHz, CDCl3) 28.0
Lead Reference
Mann; Chaplin; J.Chem.Soc.; 1937; 527-530.
Ziegler, C.B.; Heck, R.F.; J.Org.Chem.; 43; 1978; 2941-2946.
Keywords
Comments
this compound is recrystallised far more readily from hexane.
By Zac Etheridge on July 25, 2003