Formation of a vinyl epoxide from cyclohexanone
SyntheticPage 157
DOI:
Submitted: August 31, 2001, published: August 31, 2001
Authors
Rob Stockman (r.stockman@uea.ac.uk)
A contribution from
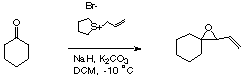
Chemicals
cyclohexanone,
sodium hydride,
potassium carbonate,
dichloromethane,
S-allyl tetrahydrothiophenium bromide
sodium hydride,
potassium carbonate,
dichloromethane,
S-allyl tetrahydrothiophenium bromide
Procedure
A mixture of sodium hydride (6 g, 0.15 mol, 60 % dispersion in mineral oil, oil removed with petroleum ether), potassium carbonate (0.6 g, 0.004 mol), and S-allyl tetrahydrothiophenium bromide (31.35 g, 0.15 mol) in dry THF (80 mL) was stirred for 10 minutes. The reaction was then cooled to -10 °C, and cyclohexanone (5 g, 0.05 mol, 5.3 mL) added. The reaction mixture was allowed to warm to room temperature, and stirred overnight. The suspension was filtered, and the filtrate was diluted with diethyl ether (100 mL). The solution was washed with brine, dried over magnesium sulfate and concentrated in-vacuo. Purification by column chromatography over silica gel (eluting with 10:1 petroleum ether/DCM then with 3:1 petroleum ether/DCM) gave the product (4g, 0.029 mol, 60 %) as a yellow oil.
Author Comments
This procedure has been carried out a few times on scales up to 10 g. The unusual combination of sodum hydride and potassium carbonate was better than other bases such as potassium tert-butoxide, KOH, DBU or either NaH or potassium carbonate on their own. Dichloromethane was a better solvent than THF of MeCN for this reaction.
Data
1H NMR (300 MHz, CDCl3), 5.71 (1H, m, vinyl), 5.40 (1H, dd, J 10.7 and 0.8, vinyl), 5.25 (1H, dd, J 17 and 0.8, vinyl), 3.13 (1H, d, J 7.2, epoxide), 1.5 (10 H, m, cyclohexane); 13C NMR (75 MHz, CDCl3), 133.2, 119.8, 64.5, 35.2, 29.5, 29.2, 25.4, 24.8, 24.5.
Lead Reference
R. W. La Rochelle, B. M. Trost and L. Krepski, J. Org. Chem. 1971, 36, 1126
Other References
F. Bourelle-Wargnier, M. Vincent and J. Chuche, J. Org. Chem., 1980, 45, 428.