Bromination of an electron-rich aromatic ring (3,5-dimethylanisole)
SyntheticPage 156
DOI:
Submitted: August 31, 2001, published: August 31, 2001
Authors
Mark Birri (mark_birri@hotmail.com)
A contribution from
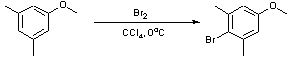
Chemicals
3,5-dimethylanisole (Avocado),
Bromine (Aldrich),
Carbon Tetrachloride (Bulk).
Bromine (Aldrich),
Carbon Tetrachloride (Bulk).
Procedure
To a solution of 3,5-dimethylanisole (26mL, 183.8mmol) in CCl4 (300mL), cooled to 0oC was added a solution of bromine (9.4mL, 183.8mmol) in CCl4 (150mL) over three hours by the aid of an addition funnel. The reaction mixture was stirred for a further 1/2 h at 0oC. The resulting orange/brown solution was quenched by addition of 3% NaOH solution (500mL). The organic layer was isolated and dried over MgSO4, filtered and concentrated in vacuo to yield a slightly yellow oil (39.5g, 100%).
Author Comments
The reaction worked well producing only mono-brominated product. Nmr analysis showed the compound to be of sufficiant purity for use without further purification, although it is possible to purify this oil by distillation. Although I myself have only carried out this reaction once, it has been used for the synthesis of the neocarzinostatin naphthoate synthesis numerous times within the group.
Data
1H nmr (300MHz, CDCl3) 2.40 (6H, s), 3.77 (3H, s), 6.66 (2H, s).
Lead Reference
Rucker, M. Brueckner, R. Synlett, 1997, 1187-1189.
Keywords
Comments
sir , i found that when this reaction carrid out at room temprature, same product was obtained but less yield and low purity, it need to purified by column chromatograpy.
By Pravin Patil on May 29, 2005