Tosylation of an alcohol
SyntheticPage 155
DOI:
Submitted: August 30, 2001, published: August 31, 2001
Authors
zac etheridge (zac.etheridge@orange.net)
A contribution from
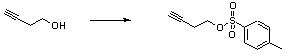
Chemicals
Pyridine,
p-toluene sulphonyl chloride,
dichloromethane (distilled from CaH2),
but-3-yn-1-ol
p-toluene sulphonyl chloride,
dichloromethane (distilled from CaH2),
but-3-yn-1-ol
Procedure
But-3-yn-1-ol (1mL, 13.2mmol) is stirred in DCM (12mL) at 0oC and p-toluene sulphonyl chloride (3.8g, 20mmol)) is added, followed by pyridine (2.2mL, 28.4mmol). The reaction is stirred at 0oC until completion (TLC, about 3 hours) then diluted with ether (35mL) and washed consecutively with water, 1M HCl, NaHCO3 (sat. soln.) and brine, dried over magnesium sulphate, filtered and concentrated. Purification is by column chromatography eluting with 15%EtOAc/PE, and yields are quantitative.
Author Comments
This is a more general procedure for tosylation than [[80|Page 80]]. It is very simple to carry out, and does not require the vast amount of pyridine used in that procedure as purification is by chromatography rather than crystallisation.
Data
1HNMR (CDCl3, 300MHz) 7.85 (2H,d), 7.39 (2H, d), 4.16 (2H, t), 2.59(2H, dt), 2.44 (3H, s), 1.95 (1H, t)
Lead Reference
G.Eglington, M.C.Whiting J.Chem.Soc. 1950 p.3650