Oximation of 2,4,6-trimethoxybenzaldehyde
SyntheticPage 146
DOI:
Submitted: August 28, 2001, published: August 29, 2001
Authors
Andrew McCarroll (andrew.mccarroll@pharminox.com)
A contribution from
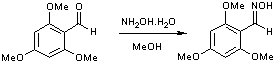
Chemicals
2,4,6-trimethoxybenzaldehyde
methanol
hydroxylamine hydrochloride
water
methanol
hydroxylamine hydrochloride
water
Procedure
To methanol (40 cm3) was added 2,4,6-trimethoxybenzaldehyde (2 g; 10.1 mmol), and the mixture stirred. Hydroxylamine hydrochloride (0.8 g; 11.5 mmol) in the minimum amount of water was added, and the mixture refluxed for 4 hours, then allowed to cool. Large amounts of water (about 1 l) were added, and the mixture allowed to stand overnight. The product precipitated, and was filtered and recrystallised (methanol) Yield 1.83 g; 86%.
Author Comments
The procedure is very simple and reliable (and old: the procedure was taken from a journal from 1903!). Syn-oxime is the sole product.
The product was prepared in a similar manner to syn-benzaldoxime and others, but with important differences (such as solvent).
Data
m.p. 207-210 (lit 201-203) 1H nmr (300 MHz, CDCl3) 3.84 (3H, s), 3.86 (6H, s), 6.14 (2H, s), 8.14 (1H,s)
Lead Reference
A. McCarroll and J. C. Walton, J. Chem. Soc., Perkin Trans 2, 2000, 1868.
Other References
J. Herzig and F. Wenzel, Monatsch. Chem. 1903, 24, 868.
Keywords
Comments
There has recently been a report describing the selective synthesis of E- or Z- oximes, with the product formed depending on the additive (CuSO~4~ gives E-oxime, K~2~CO~3~ gives Z-oxime): Sharghi and Sarvari, Synlett, 2001, 99.
By Andrew McCarroll on September 4, 2001