Mitsonobu condensation of N-hydroxyphthalimide and an alkanol.
SyntheticPage 143
DOI:
Submitted: August 28, 2001, published: August 28, 2001
Authors
Andrew McCarroll (andrew.mccarroll@pharminox.com)
A contribution from
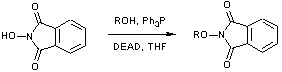
Chemicals
THF (dry)
i-propanol
N-hydroxyphthalimide
DEAD (Aldrich)
Triphenylphosphine
Ether
Sodium carbonate
i-propanol
N-hydroxyphthalimide
DEAD (Aldrich)
Triphenylphosphine
Ether
Sodium carbonate
Procedure
To THF (100 cm3) at 0 oC was added i-propanol (0.56 g; 9.25 mmol), N-hydroxyphthalimide (3.00 g; 18.5 mmol), triphenylphosphine (4.82 g; 18.5 mmol), and diethyl azodicarboxylate (3.2 cm3; 20.2 mmol) in that order. The mixture was warmed to 50 oC, and stirred at this temp for 3 days. The mixture was cooled and concentrated. Ether was added, and the solution was washed 5 times with saturated sodium carbonate solution. The aqueous layers were back extracted with ether, and the organic layers dried (MgSO4) and concentrated. The product was purified by column chromatography (PE/ether) to give colourless crystals (1.61 g; 85%)
Author Comments
The extraction is messy, due to sodium carbonate constantly crashing out of solution. The yield does not appear to be affected. When performing the column, it is better if the petrol and ether are premixed in the silica, to prevent bubbling on the column when ether meets silica for the first time. The notorious triphenylphosphine oxide was not found to be a problem.
The reaction also worked with dodecanol (69% yield) (and should work with most simple primary and secondary alcohols), but not with t-butanol, for which a different procedure needs to be used (A. Chimiak and T. Kolasa, Rocz. Chim., 1974, 48, 139.)
The reaction was not performed on any scale other than that described.
Data
m.p. 52-54 oC. 1H nmr (300MHz, CDCl3) 1.38 (6H, t, J=6.0 Hz), 4.51-4.59 (1H, septet, J=6.3Hz), 7.73-7.87 (4H,m)
Lead Reference
A. J. McCarroll and J. C. Walton, J. Chem Soc. Perkin Trans. 1, 2000, 1868-1875.
Other References
P. T. Gallagher, J. C. A. Hunt, A. P. Lightfoot and C. J. Moody, J. Chem. Soc. Perkin Trans. 2, 1997, 27, 2633.
E. Grochowski and J. Jurczak, Synthesis, 1976, 682.
A. Chimiak and T. Kolasa, Bull. Acad. Pol. Sci. Ser. Chim., 1974, 22, 195.