1,2-Addition of propargyl magnesium bromide to an enone
SyntheticPage 136
DOI:
Submitted: August 24, 2001, published: August 24, 2001
Authors
Melanie Reich (m.t.reich@sussex.ac.uk)
A contribution from
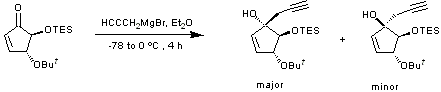
Chemicals
magnesium turnings (Avocado, 2 equiv.),
diethyl ether (anhydrous, 6 mL/mmol),
mercuric chloride (0.5 %),
propargyl bromide (Avocado, 80 wt.% in toluene, 1.9 equiv.),
cyclopentenone (prepared, 1 equiv.),
diethyl ether (distilled from sodium-benzophenone ketyl, 1 mL/mmol),
saturated ammonium chloride (1 mL/mmol),
water (1.5 mL/mmol),
brine (1.5 mL/mmol)
diethyl ether (anhydrous, 6 mL/mmol),
mercuric chloride (0.5 %),
propargyl bromide (Avocado, 80 wt.% in toluene, 1.9 equiv.),
cyclopentenone (prepared, 1 equiv.),
diethyl ether (distilled from sodium-benzophenone ketyl, 1 mL/mmol),
saturated ammonium chloride (1 mL/mmol),
water (1.5 mL/mmol),
brine (1.5 mL/mmol)
Procedure
To a suspension of magnesium turnings (744 mg, 30.56 mmol, 2 equiv.) in specially dried diethyl ether (6 mL/mmol) with mercury(II) chloride (218 mg, 0.08 mmol, 0.5 %), was added propargyl bromide (4.3 mL of an 80 wt.% solution in toluene, 29.03 mmol, 1.9 equiv.) in small portions while heating the reaction mixture at reflux (Note: A small grain of iodine is generally required to promote formation of the Grignard reagent.). The mixture was left to cool and stir at room temperature for 2 hours to give a cloudy light green solution.
The Grignard reagent was cooled to -78 ºC and a solution of the cyclopentenone (4.34 g, 15.28 mmol, 1 equiv.) in distilled diethyl ether (1 mL/mmol) was added. The mixture was allowed to stir for 4 hours slowly warming to 0 ºC. Subsequent quenching with saturated ammonium chloride solution (1 mL/mmol) was followed by extraction of the organic fraction with water (1.5 mL/mmol) and brine (1.5 mL/mmol). Drying of the organic fraction over magnesium sulfate and solvent reduction in vacuo gave the crude oil which was purified by flash column chromatography (petroleum ether/diethyl ether, 10 : 1) to give the product as a white crystalline solid (major isomer) and a clear and colourless oil (minor isomer) (4.43 g, 89 %, 19 : 1 mixture of isomers).
Author Comments
This reaction was carried out numerous times on 3 to 15 mmol of starting material, always in very good yields. Different silyl protecting groups and their effect on the stereochemical outcome (confirmed by nOe studies) of the reaction were tested. Whereas TBS (TBDMS) gives exclusively the major isomer, TES gives a 19 : 1 ratio and TMS 10 : 1. The introduction of other sterically demanding functional groups attached to the double bond, results in reduced stereoselectivity in the case of TMS, but not in the case of TBS (only one isomer obtained, see ref (a)).
With regards to formation of the Grignard reagent, it should be noted that this was carried out very dilute and utilising specially dried (BDH) diethyl ether. This reagent can also be prepared using distilled diethyl ether (sodium-benzophenone ketyl), but a higher concentration is beneficial in this case. The propargyl Grignard is easy to prepare and not particularly tricky and whatever method (and tricks) one prefers, it should work fine.
Data
1H NMR (300 MHz; CDCl3) 5.90 (1 H, dd, J 6.5 and 1.5, CH), 5.76 (1 H, dd, J 6.5 and 1.5, CH), 4.20 (1 H, dt, J 5.5 and 1.5, CHO), 3.96 (1 H, d, J 5.5, CHO), 2.62 (1 H, dd, J 17.0 and 2.5, HCH), 2.26 (1 H, dd, J 17.0 and 2.5, HCH), 2.16 (1 H, s, OH [exch]), 2.03 (1 H, t, J 2.5, CCH), 1.21 (9 H, s, (CH3)3C), 0.98 (9 H, t, J 8.0, (CH3CH2)3Si), 0.66 (6 H, q, J 8.0, (CH3CH2)3Si)
Lead Reference
(a) S. Caddick, S. Cheung, V. E. Doyle, L. M. Frost, M. G. Soscia, V. M. Delisser, M. R. V. Williams, Z. C. Etheridge, S. Khan, P. B. Hitchcock, G. Pairaudeau, S. Vile, Tetrahedron, 2001, 57, 6295; (b) S. Caddick, S. Khan, N. J. Smith, D. M. Barr, V. M. Delisser, Tetrahedron Lett., 1997, 38, 5035; (c) P. A. Wender, M. Tebbe, Tetrahedron, 1994, 50, 1419