Alkylation of zirconium chloride with neopentyl lithium
SyntheticPage 135
DOI:
Submitted: August 24, 2001, published: August 24, 2001
Authors
Colin Morton (c.morton@warwick.ac.uk)
A contribution from
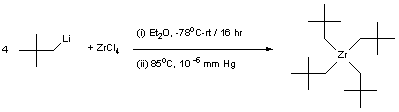
Chemicals
Diethyl ether (distilled from NaK2.2)
Neopentyl lithium (see [[120|Page 120]])
Zirconium tetrachloride (Aldrich)
Neopentyl lithium (see [[120|Page 120]])
Zirconium tetrachloride (Aldrich)
Procedure
To a large schlenk tube with stirrer bar charged with ZrCl4 (2.00 g, 8.58 mmol) and diethyl ether (50 ml) at -78oC (acetone / dry ice) was added dropwise a solution of LiCH2CMe3 (2.70 g, 37.95 mmol) in diethyl ether (50 ml) over a period of 40 min in the absence of light and with vigorous stirring. After complete addition, the reaction mixture was then surrounded with dry ice (which gradually evaporates)and was stirred overnight. The mixture had turned brown and a precipitate had formed (presumably LiCl). The mixture was then concentrated in vacuo to ca. 5 ml, transfered to a sublimation device. All volatiles were then removed and the system was attached to a turbo pump. The mixture was sublimated at 85oC (by heating the sublimation tube inside a horizontal furnace), 10-6 mm Hg, to yield an off-white solid. Yield = 2.41 g, 75 %.
Author Comments
All procedures are carried out using standard schlenk line techniques. Light is excluded by wrapping the schlenk tube in aluminium foil. A higher temperature may be required for complete sublimation but do not exceed 120oC as decomposition will occur.
Data
1H NMR (d6 benzene) 1.45 (s, 2H, CH2), 1.21 (s, 9H, CMe3).
Lead Reference
P. J. Davidson et al, J. Orgaomet., Chem., 57, 1973, 269