Deprotection of a tert-butyldimethylsilyl ether
SyntheticPage 132
DOI:
Submitted: August 24, 2001, published: August 24, 2001
Authors
Melanie Reich (m.t.reich@sussex.ac.uk)
A contribution from
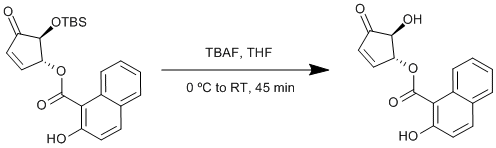
Chemicals
silyl ether (prepared, 1 equiv.),
tetrahydrofuran (distilled from sodium-benzophenone ketyl, 100 mL/mmol),
tetra-n-butylammonium fluoride (TBAF) (Aldrich, 1 M solution in THF (contains 4.5-5 % water), 1.1 equiv.),
dichloromethane (GPR, 20 mL/mmol),
water (5 mL/mmol),
brine (5 mL/mmol)
tetrahydrofuran (distilled from sodium-benzophenone ketyl, 100 mL/mmol),
tetra-n-butylammonium fluoride (TBAF) (Aldrich, 1 M solution in THF (contains 4.5-5 % water), 1.1 equiv.),
dichloromethane (GPR, 20 mL/mmol),
water (5 mL/mmol),
brine (5 mL/mmol)
Procedure
To a cold (0 ºC) solution of the silyl ether (460 mg, 1.16 mmol, 1 equiv.) in dry tetrahydrofuran (11.6 mL), was added tetra-n-butylammonium fluoride (TBAF) (1.3 mL of a 1 M solution in tetrahydrofuran, 1.27 mmol, 1.1 equiv.) and the resulting solution stirred for 45 minutes allowing the mixture to warm to room temperature. The resulting solution was diluted with dichloromethane (20 mL) and quenched with water (5 mL). The organic layer was extracted with brine (5 mL) and dried over magnesium sulfate, followed by solvent reduction in vacuo. The crude product was purified by flash column chromatography (petroleum ether/ethyl acetate, 5 : 1 to 3 : 1) to give the alcohol as a white powder (106 mg, 32 %).
Author Comments
This reaction was only carried out a couple of times, on a 100 to 500 mg scale, however only in low yields, with no starting material recovered. Decomposition and thus low yields are thought to be caused by the basicity of TBAF. Therefore buffering the reagent with acetic acid or use of buffered HF (HF.pyridine) should limit degradation and improve yields considerably. Is the compound to be deprotected not base sensitive, then TBAF is a good reagent; however, when sensitive functional groups are present a buffered reagent should be used.
Data
1H NMR (300 MHz; (CD3)2CO) 11.76 (1 H, s, OH (naph.) [exch]), 8.74 (1 H, dd, J 8.5 and 1.0, naph.), 8.10 (1 H, d, J 9.0, naph.), 7.89 (1 H, dd, J 8.5 and 1.5, naph.), 7.86 (1 H, dd, J 6.5 and 2.0, cyclop.), 7.59 (1 H, ddd, J 8.5, 7.0 and 1.5, naph.), 7.41 (1 H, ddd, J 8.5, 7.0 and 1.0, naph.), 7.22 (1 H, d, J 9.0, naph.), 6.50 (1 H, dd, J 6.5 and 2.0, cyclop.), 6.18 (1 H, q, J 2.0, cyclop.), 5.26 (1 H, d, J 5.5, OH (cyclop.) [exch]), 4.69 (1 H, dd, J 5.5 and 2.0, cyclop.)
Lead Reference
J. H. Clark, Chem. Rev., 1980, 80, 429 doi:10.1021/cr60327a004
E. J. Corey, A. Venkateswarlu, J. Am. Chem. Soc., 1972, 94, 6190 doi:10.1021/ja00772a043
E. J. Corey, A. Venkateswarlu, J. Am. Chem. Soc., 1972, 94, 6190 doi:10.1021/ja00772a043
Other References
A. B. Smith, III, G. R. Ott, J. Am. Chem. Soc., 1996, 118, 3095 (TBAF/AcOH)
K. C. Nicolaou, S. E. Webber, Synthesis, 1986, 453 (HF.py) doi:10.1055/s-1986-31673
K. C. Nicolaou, S. E. Webber, Synthesis, 1986, 453 (HF.py) doi:10.1055/s-1986-31673
Keywords
132, cyclopentenone, deprotection, hydroxy, removal, TBAF, TBDMS, TBS, tetrabutylammonium fluoride
Comments
correct page
the correct page of AB Smith paper is 13095
https://pubs.acs.org/doi/10.1021/ja963543a
By galano on December 2, 2022