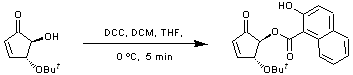
DCC coupling of an alcohol to a carboxylic acid
SyntheticPage 129
DOI:
Submitted: August 23, 2001, published: August 23, 2001
Authors
Melanie Reich (m.t.reich@sussex.ac.uk)
A contribution from
Chemicals
alcohol (prepared, 1 equiv.),
carboxylic acid (Lancaster, 1.2 equiv.),
tetrahydrofuran (distilled from sodium-benzophenone ketyl, 5 mL/mmol),
dichloromethane (distilled from CaH, 5 mL/mmol),
1,3-Dicyclohexylcarbodiimide (DCC) (Avocado, 1.3 equiv.)
carboxylic acid (Lancaster, 1.2 equiv.),
tetrahydrofuran (distilled from sodium-benzophenone ketyl, 5 mL/mmol),
dichloromethane (distilled from CaH, 5 mL/mmol),
1,3-Dicyclohexylcarbodiimide (DCC) (Avocado, 1.3 equiv.)
Procedure
The alcohol (1.02 g, 5.99 mmol, 1 equiv.) and 2-hydroxy-1-naphthoic acid (1.35 g, 7.12 mmol, 1.2 equiv.) were dissolved in dry tetrahydrofuran (30 mL/mmol) and dry dichloromethane (30 mL/mmol) and the solution cooled to 0 ºC. 1,3-Dicyclohexylcarbodiimide (DCC) (1.60 g, 7.79 mmol, 1.3 equiv.) was added, in portions, over 30 minutes and the solution allowed to stir at 0 ºC for 5 minutes. The resulting suspension was filtered through celite and the solvent reduced in vacuo. Purification by flash column chromatography (petroleum ether/ethyl acetate, 5 : 1) yielded the desired product as a white solid (2.01 g, 99%).
Author Comments
This transformation has been carried out numerous times, on 50 mg to 1 g, always achieving excellent yields.
Whereas usually the carboxylic acid is mixed with DCC to form the active ester, before addition of the alcohol, this is not possible in this case as the hydroxyl group on the naphthoic acid will result in formation of dimers. Hence, both starting materials are dissolved in THF/DCM before addition of the reagent. Furthermore, it should be noted that these types of transformation usually proceed best in dichloromethane, but had to be carried out in a mixture of THF and DCM, because of limited solubility of the carboxylic acid.
The side-product from this reaction, a urea, is partly soluble in aqueous as well as organic phases and thus purification by column chromatography is necessary. This can be avoided by employing alternative, more expensive reagents such as EDC (1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, moderate yields) and DIC (diisopropylcarbodiimide, yields almost as high as with DCC).
Data
1H NMR(300 MHz; (CD3)2CO) 11.75 (1 H, s, OH [exch]), 8.72 (1 H, app d, J 9.0, H-9 (naph.)), 7.92 (1 H, d, J 9.0, H-4 (naph.)), 7.76 (1 H, app d, J 8.0, H-6 (naph.)), 7.56 (1 H, ddd, J 9.0, 7.0 and 1.5, H-8 (naph.)), 7.43 (1 H, dd, J 6.0 and 2.0, H-3 (cyclop.)), 7.38 (1 H, ddd, J 8.0, 7.0 and 1.0, H-7 (naph.)), 7.17 (1 H, d, J 9.0 H-3 (naph.)), 6.41 (1 H, dd, J 6.0 and 2.0, H-2 (cyclop.)), 5.45 (1 H, d, J 2.0, H-5 (cyclop.)), 5.11 (1 H, q, J 2.0, H-4 (cyclop.)), 1.25 (9 H, s,(CH3)3C)
Lead Reference
(a) Smith, Moffatt, Khorana, J. Am. Chem. Soc., 1958, 80, 6284; (b) Balcom, Petersen, J. Org. Chem., 1989, 54, 1922 ;(c) Takahashi, Toshiyuki, Suzuki, Hirama, Tetrahedron, 1994, 50, 1327
Keywords
Comments
chemistry
How is TLC appeared?
By lavanya on September 30, 2019