Oxidation of an acetylenic alcohol
SyntheticPage 127
DOI:
Submitted: August 23, 2001, published: August 23, 2001
Authors
Melanie Reich (m.t.reich@sussex.ac.uk)
A contribution from
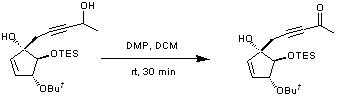
Chemicals
Dess-Martin periodinane (for preparation see [[51|Page 51]], 3 equiv.),
dichloromethane (distilled from calcium hydride, 2 x 5 mL/mmol),
alcohol (1 equiv.),
aqueous sodium thiosulfate solution (5 mL/mmol),
diethyl ether (GPR, 15 mL/mmol),
ethyl acetate (GPR, 15 mL/mmol),
saturated sodium hydrogen carbonate solution (5 mL/mmol),
brine (5 mL/mmol)
dichloromethane (distilled from calcium hydride, 2 x 5 mL/mmol),
alcohol (1 equiv.),
aqueous sodium thiosulfate solution (5 mL/mmol),
diethyl ether (GPR, 15 mL/mmol),
ethyl acetate (GPR, 15 mL/mmol),
saturated sodium hydrogen carbonate solution (5 mL/mmol),
brine (5 mL/mmol)
Procedure
To a suspension of 1,1,1-triacetoxy-1,1-dihydro-1,2-benziodoxol-3(1H)-one (DMP)(1.19 g, 2.79 mmol, 3 equiv.) in dry dichloromethane (5 mL) was added a solution of the starting material (342 mg, 0.93 mmol, 1 equiv.) also in dichloromethane (5 mL). The resulting white suspension was allowed to stir for 30 minutes at room temperature after which it was quenched with aqueous sodium thiosulfate solution (5 mL) and extracted with diethyl ether (15 mL) and ethyl acetate (15 mL). The organics were washed with saturated sodium hydrogen carbonate solution (5 mL) and brine (5 mL), dried over magnesium sulfate and the solvent removed in vacuo. The crude product was purified by flash column chromatography (petroleum ether/diethyl ether, 3 : 1) to yield the desired ketone as a yellow solid (310 mg, 91 %).
Author Comments
This reaction was carried out a couple of times, on a 1 mmol scale. The same transformation was carried out on analogous compounds where the TES protecting group was replaced with TMS (slightly lower yield) and TBS, both of which worked well.
The numbering in the 1H NMR is counterclockwise starting with the quaternary carbon.
Data
1H NMR (300 MHz; CDCl3) 5.87 (1 H, dd, J 6.5 and 1.0, H-2), 5.81 (1 H, dd, J 6.5 and 1.0, H-3), 4.26 (1 H, dt, J 5.5 and 1.0, H-4), 4.00 (1 H, d, J 5.5, H-5), 2.72 (1 H, d, J 17.5, HCH), 2.49 (1 H, d, J 17.5, HCH), 2.32 (3 H, s, CH3), 1.22 (9 H, s, (CH3)3C), 0.98 (9 H, t, J 8.0, (CH3CH2)3Si), 0.66 (6 H, q, J 8.0, (CH3CH2)3Si)
Lead Reference
(a)Caddick S, Khan S, Frost LM, Smith NJ, Cheung S, Pairaudeau G; Tetrahedron, 2000, 56, 8953-8958; (b) Ireland, R.E.; Liu, L.; J. Org. Chem., 1993, 58, 2899; (c) D. B. Dess, J. C. Martin, J. Am. Chem. Soc., 1991, 113, 7277; (d) Dess, D.B.; Martin, J.C.; J. Org. Chem., 1983, 48, 4156