Dess-Martin Oxidation of Allylic Alcohols
SyntheticPage 126
DOI:
Submitted: August 23, 2001, published: August 23, 2001
Authors
zac etheridge (zac.etheridge@orange.net)
A contribution from
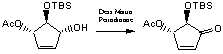
Chemicals
Dess Martin periodinane
Dicholoromethane (distilled from CaH2)
trans, trans-3-Acetoxy-5-hydroxy-4-tbutyldimethylsiloxy-cyclopentene
Dicholoromethane (distilled from CaH2)
trans, trans-3-Acetoxy-5-hydroxy-4-tbutyldimethylsiloxy-cyclopentene
Procedure
The alcohol (40mg, 0.15mmol) is stirred at room temperature in DCM (3mL) under an inert atmosphere and the periodinane (99mg, 0.236mmol) is added in one portion. The reaction is stirred until completion (TLC, about 2 hours) then quenched with sodium thiosulphate (saturated solution), extracted 4 times with ethyl acetate, washed wiht brine, dried over magnesium sulphate, filtered and concentrated in vacuum. The product is purified by column chromatogrpahy using a short column (very little impurity) eluting with 30% EtOAc/PE to yield about 36mg (90%) of colourless oil.
Author Comments
The Dess-Martin oxidation is very simple and versatile, and also tolerant of most functional groups. The oxidant can be made [[51|Page 51]] or purchased from Lancaster.
Data
1H NMR (300MHz, CDCl3) 7.23 (1H, dd, J=1.7, 6.4), 6.17 (1H, d, J=6.0), 5.54 (1H, m), 4.15 (1H, d, J=2.6), 2.01 (3H, s), 0.79 (9H, s), 0.04 (3H, s), 0.00 (3H, s).
Lead Reference
S.Caddick et al Tetrahedron 2001 p6295