Chlorination of Uranium Oxide with hexachloropropene
SyntheticPage 122
DOI:
Submitted: August 22, 2001, published: August 22, 2001
Authors
Peter Scott (peter.scott@warwick.ac.uk)
A contribution from
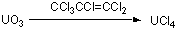
Chemicals
UO3 (orange powder supplied by BNFL; see also refs)
Hexachloropropene (Aldrich)
Dichloromethane (distilled from CaH2 or otherwise dried)
Hexachloropropene (Aldrich)
Dichloromethane (distilled from CaH2 or otherwise dried)
Procedure
The U material is radioactive so please follow your local rules. The reaction is performed under N2 or Ar using a Schlenk line in a fumehood.
Place the orange oxide (40 g, 0.14 mol) and hexachloropropene (100 ml, ca. 0.71 mol) in a 2-neck 1 litre round-bottom flask (500 ml is also possible) with the biggest magnetic follower you can find. We use an egg-shaped one. Fit an efficient condenser and thermometer such that the thermometer reads the temperature of the suspension. You will need a heating mantle through which a magnetic stirrer will operate. Make sure it's a good one like a Heidolph. We have also used a silicone or Lutron oil-bath. Heat the mixture slowly to about 60oC with gentle stirring. At about that temperature a fairly vigorous exotherm usually occurs, hence the use of an efficient condenser (see comments). Keep below 100oC and cool if necessary. After the exotherm subsides, heat the suspension to reflux (158-160oC) for 6 h. If you can't heat to this temperature you will probably need to heat for longer. The red solid gradually turns to a new green precipitate. After cooling, isolate this solid material by filtration as follows. Transfer the solid through a wide-bore cannula to a frit using dichloromethane to wash it over. Wash thoroughly with dichloromethane (at least 3 x 30 ml until the washings run pale or colourless). Dry the solid cautiously in vacuo on the sinter so that it is easier to scrape out. Transfer it to a Schlenk and dry in vacuo again at ambient temperature for an hour or so. Then heat in vacuo at 160oC for 24 h to remove any further volatiles. You should be left with the leaf-green free-flowing solid UCl4 (51 g, 96%)
Author Comments
The stirring is probably important at the start but once things get going we have found that it's hard to stir, not least because of the high density. Don't worry too much as long as it's moving about.
On a couple of occasions we did not get the exotherm as described above. In these cases we found that heating for an hour at about 60 oC before cautiously raising the temperature led to the same product. Occasionally also the thing is sufficiently exothermic to reflux the system quite vigorously.
We store the material in Nalgene bottles in a glovebox.
The literature method uses twice as much hexachloropropene which is expensive, as well as being unpleasant and hazardous. Our method also uses CH2Cl2 instead of CCl4 which many prefer not to handle.
We have performed the reaction many times on this scale and it is very reliable.
Data
Microanalysis might be useful but we have never done this. We work on the basis of our experience that the bright green free-flowing stuff gives nearly quantitative yields in certain standard reactions.
Lead Reference
J. A. Hermann and J. F. Suttle, Inorg. Synth., 1957, 5, 143