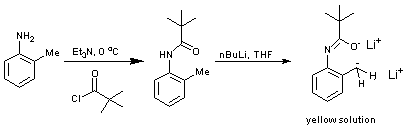
N-pivaloylation of an amine
SyntheticPage 107
DOI:
Submitted: August 20, 2001, published: August 20, 2001
Authors
lisa frost (lisa.frost@evotec.com)
A contribution from
Chemicals
o-toluidine (1 equiv)
triethylamine (1 equiv)
pivaloyl chloride (1 equiv)
dichloromethane (0.6 mL/ mmol)
n-butyl lithium (1 equiv)
THF (5 mL/ mmol)
triethylamine (1 equiv)
pivaloyl chloride (1 equiv)
dichloromethane (0.6 mL/ mmol)
n-butyl lithium (1 equiv)
THF (5 mL/ mmol)
Procedure
Procedure a: To a stirred solution of o-toluidine (10 g) and triethylamine (9.41 g) [not necessarily dry] in anhydrous DCM (50 mL) cooled to 0 oC was slowly added a solution of pivaloyl chloride (11.2 g) in anhydrous DCM (c.a. 10 mL/ 100 mmol). Upon complete addition stirring was continued for a further 30 minutes and then poured into water (200 mL). The organic layer was washed with water (3 x 100 mL) and dried over sodium sulphate. The crude white solid afforded on solvent evaporation was recrystallised twice from hot DCM / hexane (12/80 mL) to afford the title compound as a white solid. Yield 83 %.
Procedure b: To a solution of N-pivaloyl-o-toluidene (70-100 mg) in anhydrous THF (c.a. 1 mL) in a flame dried, nitrogen flushed flask was slowly added n-BuLi [microsyringe] until a colour change was evident [bright yellow].
Author Comments
The indicator can be formed on a 10 g scale and is indefinitely air stable. The reference also identifies another indicator which can be prepared in an analogous fashion [N-pivaloyl-o-benzylaniline] and can again be used for titration of organolithium bases. These indicators are widely used for the titration of nBuLi but also show a colour change for tBuLi, sBuLi, MeLi and PhLi. On a practical point, the titration must be carried out under inert conditions or a false end-point may occur [i.e. yellow colour may disappear]. Also, the end-point is more readily observed if a filter paper is placed under the flask. Finally, the titration should be carried out three times, or until two concurrent results so as to receive an accurate molarity.
Data
m.p 109-111 oC, 1H nmr (300 MHz, CDCl3) 1.34 (9H, s), 2.27 (3H, s), 7.07 (1H, dd, J=7.5, 1.1Hz), 7.17 (1H, dd, J=7.5, 0.5Hz), 7.22 (1H, d, J=7.5Hz), 7.87 (1H, d, J=7.5Hz).
Lead Reference
Jean Suffert; J. Org. Chem. 1989, 54, 509-510