Palladium catalysed coupling of a vinyl iodide with a terminal alkyne (Sonogashira coupling)
SyntheticPage 106
DOI:
Submitted: August 17, 2001, published: August 17, 2001
Authors
Marco Soscia (m.g.soscia@sussex.ac.uk)
A contribution from
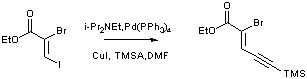
Chemicals
Hunigs base (aldrich)
Pd(PPh3)4
CuI (avocado)
TMSA (aldrich)
DMF (distilled from CaH2)
Pd(PPh3)4
CuI (avocado)
TMSA (aldrich)
DMF (distilled from CaH2)
Procedure
To a deoxygenated solution of iodide (10 g, 33 mmol, 1 equiv.) in DMF (62 mL) cooled to 0oC was added diisopropylethyl amine (6.4 g, 49 mmol, 1.5 equiv.) and TMS acetylene (3.2 g, 33 mmol, 1 equiv.). The reaction mixture was seen to lighten in colour (orange to yellow). Copper iodide (280 mg, 1.48 mmol, 4.5 mol %) and Pd(PPh3)4 (758 mg, 0.66 mmol, 2 mol %) were added and stirring continued overnight at 0oC. The reaction was quenched with NH4Cl solution (40 mL) and extracted into ether (5 x 120 mL). The combined organics were washed with water (20 mL), dried (MgSO4), filtered and concentrated in vacuo leaving a brown oil. Purification of this oil was achieved by chromatography on silica gel with petrol:ether (20:1 v:v) a the eluent yielding the title product as a yellow oil (6.84 g, 76 %).
Author Comments
Due to the reactivity of Pd(PPh3)4 it was freshly prepared prior to use and never kept for longer than a few months in the freezer as a dark colouration proceeds signifying decomposure. The reaction reaction has been scaled up to 10g with no problems.
Data
1H: 7.3 (s, 1H), 4.3 (q, 2H, J=7.12Hz), 1.35 (t, 3H, J=7.12Hz), 0.26 (s, 9H)
Lead Reference
Myers PD, Carter R, Davies M, Elliot J, Pitterna T, Tetrahedron, 1996; 72: 104
Other References
S.Caddick, V.M.Delisser, V.E.Doyle, S.Khan, Tetrahedron,1999, 2737-2754