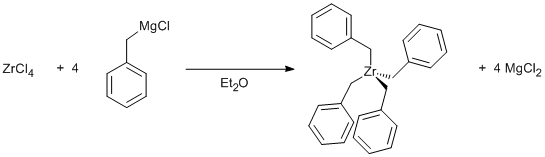
Alkylation of zirconium tetrachloride with benzyl Grignard
SyntheticPage 1
DOI:
Submitted: May 30, 2001, published: June 1, 2001
Authors
Peter Scott (peter.scott@warwick.ac.uk)
A contribution from
Chemicals
C6H5CH2MgCl (1M in diethyl ether, Aldrich)
ZrCl4 (not sublimed)
diethyl ether (dried over Na/K but Na would do)
toluene (dried over Na)
ZrCl4 (not sublimed)
diethyl ether (dried over Na/K but Na would do)
toluene (dried over Na)
Procedure
C6H5CH2MgCl (100 ml, 1M in diethyl ether, 4 equiv.) was added to ZrCl4 (6.0 g, 0.025 mol) in a diethyl ether (50 ml) slurry over 1 hr at -78°C. Make sure the suspension is cool before you start adding the Grignard reagent. After stirring overnight (in the absence of light) the bright orange solution was cooled to 0°C and filtered to remove MgCl2 [this residue should be "destroyed" by cautious hydrolysis before disposal]. The diethyl ether was removed in vacuo and the orange product was redissolved in toluene (30 ml) and filtered. Cooling to -30°C afforded orange crystals which were washed with cold toluene (2 x 10 ml). Yield variable, but about 70%.
Author Comments
Keeping the reaction in the dark is important. Use aluminium foil. Sometimes the yield from the first crop is low and a second crop can be obtained by concentration and cooling. We store the product in the freezer in a Schlenk wrapped in foil and transfer the Schlenk to the glovebox to handle it. If you have a freezer in the box that is ideal. Don't forget that this type of method will only work for alkyls without beta-H atoms (beta elimination).
Data
1H NMR (293 K, d6-benzene) 7.15 (m, 12H, Ph), 6.42 (d, 8H, Ph), 1.57 (s, 8H, CH2).
Lead Reference
U. Zucchini, E. Albizatti and U. Gianni, J. Organomet. Chem., 1971, 26, 357.
Keywords
Comments
The reaction is conducted with rigorous exclusion of air and water (Schlenk Procedures)
By Max Hammond on February 16, 2002
We have generally used e.g. Aldrich ZrCl4 (99.5+) for this reaction. Note that if you are unsure of the purity of the ZrCl4 you should source some clean material or sublime some. ZrCl4 is pretty air sensitive, so if your jar is on a shelf in the lab then it is probably mostly "ZrCl2O" etc.. It would be inetresting to hear if the THF adduct ZrCl4(THF)2 is good material to use. It might be OK because the reaction is performed in ether.
By Peter Scott on April 19, 2005
I suppose you could use the THF adduct, but I have used the ether adduct ZrCl4(OEt2)2 in this prep in the past. The ether adduct is prepared the same way as the THF analogue
By Andrew Gott on April 29, 2005
I have tried to use benzyl magnesium chloride 2.0M in THF but it didn't work properly. I then discovered that THF can coordinate to the zirconium tetrabenzyl and the resulting adduct is insoluble in toluene (J. Organomet. Chem., 1974, 74, pag 417).
By Sara Ronca on June 29, 2009
THF
Since this time we have made Zr tetrabenzyl by this method many times using ZrCl4 and the Grignard in ether.
By Peter Scott on March 9, 2010
stirring over night
Do you leave the reaction over night at -78C as well? or you let it reach to R.T, and then when u want to filter you cool it again to 0C?
By dana on October 23, 2012
The method described cooling to 0oC, so no it was not stirred at -78 overnight. Nevertheless, the method has now been clarified.
By Peter Scott on October 23, 2012