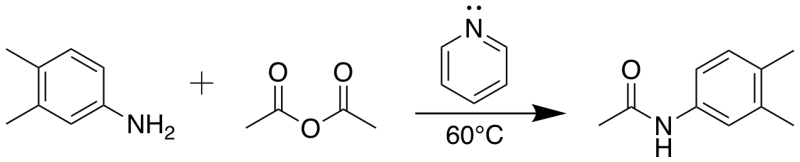
Amidation of acetic anhydride with 3,4-dimethylaniline
SyntheticPage 931
Submitted: September 9, 2020, published: September 22, 2020
Authors
Steven Kennedy (Steven.Kennedy@millersville.edu)
Yongyu Ou (yoou@millersville.edu)
A contribution from
Chemicals
3,4-dimethylaniline (>99.0%, TCI America)
Acetic anhydride (97+%, Alfa Aesar)
Pyridine (99.0%, EMD Millipore)
Hexane (99%, VWR Chemicals)
Ethyl acetate (99%, Alfa Aesar)
Ammonium chloride (Fisher Scientific)
Magnesium sulfate (EMD Millipore)
Procedure
3,4-dimethylaniline (2.4 g, 20 mmol) was dissolved in pyridine (36 mL) and acetic anhydride (9.4 mL, 100 mmol) was added at room temperature. The reaction was stirred at 60 °C for 2 hours. The solvent was removed in vacuo. The crude product was extracted with ethyl acetate (4 X 50 mL) and washed with saturated aqueous ammonium chloride solution (60 mL). The combined ethyl acetate layers were dried over anhydrous magnesium sulfate, filtered, and concentrated by rotary evaporation, leaving a white solid product (3.15 g, 96.6%).
Author Comments
Data
Rf = 0.12 (1:2 EtOAc/Hex)
1 H NMR (400 MHz, CDCl3): δ 7.27 (m, 1H), 7.20 (d, J = 8.0 Hz, 1H), 7.06 (d, J = 8.4 Hz, 1H), 2.22 (d, J = 8.4 Hz, 6H), 2.15 (s, 3H)
13 C NMR (100 MHz, CDCl3): δ 168.2, 137.2, 135.6, 132.6, 129.9, 121.4, 117.5, 24.5, 19.9, 19.2
Lead Reference
Guo, Liangqin; He, Shuwen; et al. Preparation of Cycloalkylcarbonyl or heterocycloalkylcarbonyl-substituted spiropiperidines as melanocortin-4 receptor agonists for the treatment of conditions such as Obesity. Patent WO 2004089307 A2, October 21, 2014.
Supplementary Information
Keywords
amide formation; nucleophilic acyl substitution, amides, amines, aromatics/arenes, substitution