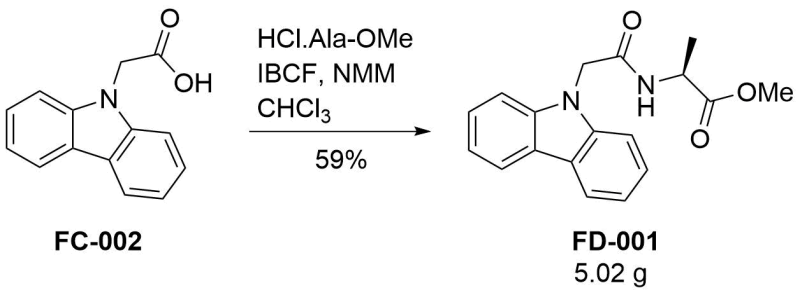
Condensation of [[925|2‐(9H‐carbazol‐9‐yl)acetic acid]] and L-alanine methyl ester
SyntheticPage 926
Submitted: April 29, 2020, published: May 4, 2020
Authors
Bart Dietrich (bart.dietrich@yahoo.co.uk)
A contribution from
Chemicals
L-Alanine methyl ester hydrochloride (Fluorochem)
Isobutyl chloroformate (Fluorochem)
N-Methylmorpholine (Fluorochem)
Chloroform (Fisher)
Procedure
To a suspension of [[925|2‐(9H‐carbazol‐9‐yl)acetic acid]] (6.12 g, 27.2 mmol) in chloroform (50 mL) were added isobutyl chloroformate (1.05 eq, 3.70 mL) and N-methylmorpholine (1.1 eq, 3.29 mL). After stirring for 10 minutes, L-alanine methyl ester hydrochloride (1.1 eq, 4.17 g) and another portion of N-methylmorpholine (1.1 eq, 3.29 mL) were added and the reaction was stirred overnight. The reaction mixture was diluted with chloroform, washed in turn with 1M hydrochloric acid (2×), brine, dried (MgSO4), and evaporated under reduced pressure. The crude material was recrystallised from boiling ethanol. The room-temperature crop presented as fluffy crystals, while another crop of a denser solid was obtained after cooling the mother liquor from the first crop in a freezer. Both crops were of sufficient NMR purity for the next step and were combined. Total yield: 5.02 g (59 %).
Author Comments
- Effervescence is observed on adding the alanine methyl ester, before even the second equivalent of NMM is added. Presumably the carbazole nitrogen is acting as a base here, mopping up the HCl from the alanine ester hydrochloride and allowing the alanine ester to react with the mixed anhydride, kicking out CO2.
- A slight exotherm is observed during the reaction. The reaction may be run under ice/water cooling.
Data
dH (400 MHz, DMSO-d6) 8.87 (1H, d, J 7.18, NH), 8.14 (2H, d, J 7.64, HAr), 7.52 (2H, d, J 8.22, HAr), 7.43 (2H, ddd, J 8.22, 7.08, 1.14, HAr), 7.20 (2H, ddd, J 7.85, 6.98, 0.89, HAr), 5.12 (1H, d, J 16.84, NCHaHb), 5.02 (1H, d, J 16.84, NCHaHb), 4.32 (1H, pseudo-quintet, J 7.25, CH*), 3.61 (3H, s, OCH3), 1.34 (3H, d, J 7.28, CH*CH3). dC (100 MHz, DMSO-d6) 172.79 and 167.47 (C=O), 140.61, 125.61, 122.23, 120.09, 118.98, and 109.33 (CAr), 51.88 (OCH3), 47.70 (CH*), 45.22 (NCH2), 17.04 (CH*CH3). HRMS (ESI) m/z: [M+Na]+ calcd for C18H18N2NaO3 333.1210; found 333.1206.
Keywords
amino acids, aromatics/arenes, carbazole, condensation, esters, heterocyclic compounds