Nitration of 2,3,3-trimethylindolenine to produce 5-nitro-2,3,3-trimethylindolenine
SyntheticPage 911
Submitted: December 14, 2019, published: December 31, 2019
Authors
Matthew C Jackson
Robert Smith (rbsmith@uclan.ac.uk)
A contribution from
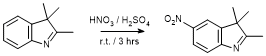
Chemicals
2,3,3-Trimethylindole (Alfa Aesar)
Sulphuric acid (Fisher Scientific)
Nitric Acid (Fisher Scientific)
Procedure
To a 250 mL wide necked conical flask* was added 2,3,3-trimethylindole (12.42 g, 78 mmol) and concentrated sulphuric acid (46 mL). The solution was transferred to an ice bath and with constant string a pre-cooled mixture of concentrated sulphuric acid (7 mL) and concentrated nitric acid (9.4 mL, 0.23 mol) was added dropwise via a dropping funnel. Upon total addition of the acidic mixture, the reaction was allowed to stir at room temperature for 3 hours. The reaction mixture was then poured over ice (approx. 500 g) which yielded a red precipitate. Upon standing overnight, the pH was then adjusted to pH=8, via the slow addition of sodium hydroxide pellets** and the precipitate produced unpon neutralisation was isolated by vacuum filtration and washed with deionised water (500 mL). The solid was dissolved in a minimum amount of hot methanol, filtered*** and evaporated to dryness to afford 2,3,3-trimethyl-5-nitro-indole (11.93 g, 78%) as a red solid****
Author Comments
*A conical flat bottomed flask fitted with a dropping funnel was used. Stirring was accomplished using a stirring bar, which was controlled from the hot plate. The whole reaction was accomplished in the open air without the need of dry/inert conditions.
** Universal indicator paper was used to monitor the change in pH to pH=7.
*** Filtration was used to remove any impurities.
**** The red solid can be can be recrystallised to give a pure compound, however we wanted to reduce the nitro to the amine and were happy to proceed with the crude NMR which is shown with minor impurities.
Data
1H-NMR (300 MHz, Chloroform-d): δ 8.25 (dd, J = 8.5, 2.2 Hz, 1H, Ar-H), 8.16 (d, J = 2.1 Hz, 1H, Ar-H), 7.61 (d, J = 8.5 Hz, 1H, Ar-H), 2.36 (s, 3H, C-CH3) 1.37 (s, 6H, 2C-CH3). 13C-NMR (300 MHz, Chloroform-d): δ 194.24, 158.95, 146.69, 145.61, 124.56, 120.05, 117.19, 54.51, 22.74, 16.02. IR (ATR): 2977, 1565, 1515, 1420, 1389, 1333, 1206, 1112, 1056, 916, 847, 803, 738. GC-MS (EI) m/z: 91.02, 114.98, 143.05, 189.00, 204.05 ([M-H]+).
Lead Reference
Santos, P.F., Reis, L.V., Duarte, I., Serrano, J.P., Almeida, P., Oliveira, A.S., Ferreira, L.F.V. Synthesis and photochemical evaluation of iodinated squarylium cyanine dyes (2005) Helvetica Chimica Acta, 88 (5), pp. 1135-1143.
Keywords
aromatics/arenes, heterocyclic compounds, nitration