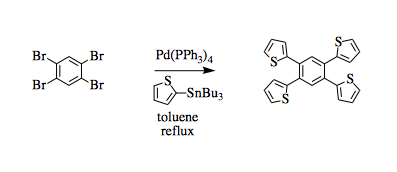
Synthesis of a tetrathiophenylbenzene by Stille coupling
SyntheticPage 871
DOI:
Submitted: December 5, 2018, published: January 1, 2019
Authors
Kathryn Allen (kathryn.allen@millersville.edu)
Liam Schroeder (lfschroe@millersville.edu)
A contribution from
Chemicals
Toluene, anhydrous, 99.8% Aldrich
1,2,4,5-tetrabromobenzene, 97% Aldrich
2-(tributylstannyl)thiophene, 97% Aldrich
tetrakis(triphenylphosphine)Palladium(0), 99% Aldrich
1,2,4,5-tetrabromobenzene, 97% Aldrich
2-(tributylstannyl)thiophene, 97% Aldrich
tetrakis(triphenylphosphine)Palladium(0), 99% Aldrich
Procedure
A three-necked round-bottomed flask was equipped with a condenser, three septa, and a stir bar. The flask was purged with nitrogen gas for 30 minutes. Anhydrous toluene (75 mL) was added under nitrogen. 1,2,4,5-Tetrabromo benzene (2.5 g, 6.35 mmol, 1 eq.) and 2-(tributylstannyl)thiophene (10 mL, 31.75 mmol, 5 eq.) were added. A catalytic amount (spatula tip) of tetrakis(triphenyl phosphine)palladium(0) was added and the reaction was allowed to reflux at 120°C for 16 hours. Completion of the reaction was monitored through TLC (10% ethyl acetate/hexanes, product Rf = 0.51). The reaction was taken off heat and the slushy was dissolved completely in ethyl acetate. The organic layer was washed two times with 10% sodium hydroxide solution ( 200 mL) to remove tin and then dried over sodium sulfate, filtered, and concentrated under reduced pressure on a rotary evaporator to yield the crude product. The material was directly recrystallized from 25% ethyl acetate in hexanes to give grey-tan product (2.28 g, 5.61 mmol, 88%).
Author Comments
The Stille is exceptionally robust to moisture and air. However, all glassware was treated in a base bath after the Stille reaction and the Stille waste was segregated in its own waste container to remove tin by-products. Organotin reagents are toxic and should be handled with correct PPE under the supervision of an experienced chemist.
The authors thank Dr. Daniel Ralston for DART MS collection.
The authors thank Dr. Daniel Ralston for DART MS collection.
Data
1H NMR (400 MHz, CDCl3): δ 7.664 (s, 2H), 7.303 (m, 4H), 6.974 (m, 8H).
13C NMR (400 MHz, CDCl3): δ 141.764, 133.565, 133.421, 127.583, 127.204, 126.518.
Mass Spec: Calculated 406.5940 g/mol; Observed 407.0079 g/mol
13C NMR (400 MHz, CDCl3): δ 141.764, 133.565, 133.421, 127.583, 127.204, 126.518.
Mass Spec: Calculated 406.5940 g/mol; Observed 407.0079 g/mol
Lead Reference
Chem. Mater., 2008, 20 (7), pp 2484–2494.
Keywords
aromatics/arenes, heterocyclic compounds, Stille coupling