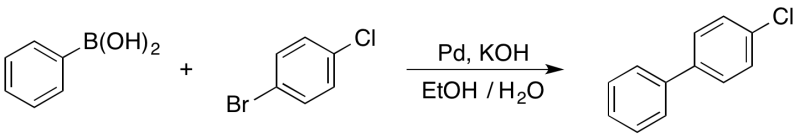
Suzuki-Miyaura Cross-Coupling of phenylboronic acid and 4-bromochlorobenzene
SyntheticPage 822
DOI:
Submitted: June 14, 2017, published: June 15, 2017
Authors
Gloria S. Chung (gschung@millersville.edu)
Michael D. Buell (mdbuell@millersville.edu)
Steven M. Kennedy (Steven.Kennedy@millersville.edu)
A contribution from
Chemicals
Phenylboronic acid (98% Combi-Blocks)
Palladium atomic absorption standard solution, 1,000 μg/mL Pd in 5% HCl (Sigma-Aldrich)
95% Ethanol
1M Potassium hydroxide in water
Dichloromethane (DCM)
Dimethyl sulfone (DMSO2, a Standard for quantitative NMR, TraceCERT®, Sigma-Aldrich)
Procedure
Open to air, in a 25 mL round bottom flask, 4-bromochlorobenzene (0.189 g, 1.0 mmol) was completely dissolved in 8 mL of 95% ethanol by rapid magnetic stirring; next, phenylboronic acid (0.128 g, 1.05 mmol) was added, followed by 2 mL of 95% ethanol. The palladium standard solution (approx. 0.2 mL) was added via a 1 mL syringe. The resulting solution was stirred for 3 minutes before 2 mL of 1 M potassium hydroxide solution was added. The reaction mixture was stirred rapidly at room temperature for 25 minutes; at which time, ice cold water (5 mL) was added. After collection via vacuum filtration, the solid crude product was washed with ice-cold water (2 mL). The crude product was dissolved in DCM (2 mL); then, the solution was passed through a plug of anhydrous magnesium sulfate using an additional 4 mL of DCM. The NMR internal standard (IS), dimethyl sulfone (0.047 g, 0.5 mmol), was added to the DCM solution (approx. 6 mL). The DCM was removed via rotory evaporation to yield 4-chloro-1,1'-biphenyl as a white powder (0.12 g, 0.64 mmol, 64% isolated yield, 58% yield based on IS).
Author Comments
Data
13 C NMR (100 MHz, CDCl3): 139.96, 139.64, 133.34, 128.90, 128.87, 128.38, 127.58, 126.97.
Lead Reference
Other References
Keywords
aromatics/arenes, ligand-free, organometallics, palladium