Regioselective Diels-Alder Reaction of 5-hydroxy-1,4-naphthoquinone with Isoprene at Room Temperature
SyntheticPage 800
DOI:
Submitted: October 13, 2015, published: October 19, 2015
Authors
Andrew J. Smaligo (andrew.smaligo@gmail.com)
Jevica Salim (jev340@gmail.com)
Steven M. Kennedy (steven.kennedy@millersville.edu)
A contribution from
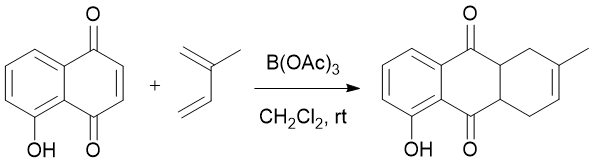
Chemicals
5-hydroxy-1,4-naphthoquinone (Alfa Aesar)
Isoprene (Sigma-Aldrich)
Boron triacetate
Dichloromethane (Alfa Aesar)
Isoprene (Sigma-Aldrich)
Boron triacetate
Dichloromethane (Alfa Aesar)
Procedure
To a stirred solution of 5-hydroxy-1,4-naphthoquinone (2.907 g, 12 mmol) in dichloromethane (160 mL) was added boron triacetate (2.481 g, 13.2 mmol) at room temperature. The reaction mixture was sealed and allowed to stir for approximately fifteen minutes. Isoprene (3.60 mL, 36 mmol) was then added to the flask via syringe. Upon completion (indicated by TLC), the reaction was quenched by addition of saturated aqueous sodium bicarbonate (160 mL). The aqueous layer was extracted with dichloromethane (4 x 100 mL), and the combined organic fractions were washed with water (3 x 100 mL), brine (100 mL), dried over anhydrous magnesium sulfate, and passed through a celite filter. Removal of the solvent by rotary evaporation yielded the product (5-hydroxy-2-methyl-1,4,4a,9a-tetrahydroanthracene-9,10-dione) as a dark-yellow solid (5.483 g, 94%).
Author Comments
The dienophile solution turns a blood-red color upon addition boron triacetate, and changes to a dark-brown color upon addition of isoprene. The color changes described may be instantaneous or delayed.
A small amount of the undesired regioisomer is formed by this reaction. The regioselectivity of this reaction determined by 1H NMR spectrum analysis was 8:1 (see supplementary information). This reaction has been performed from 1 mmol to 12 mmol scale. Each reaction resulted in high yield and good regioselectivity.
A small amount of the undesired regioisomer is formed by this reaction. The regioselectivity of this reaction determined by 1H NMR spectrum analysis was 8:1 (see supplementary information). This reaction has been performed from 1 mmol to 12 mmol scale. Each reaction resulted in high yield and good regioselectivity.
Data
1H NMR (400 MHz, CDCl3): 1.72 (s, 3H), 2.15 (d, 1H), 2.32 (d, 1H), 2.42 (d, 1H), 2.56 (d, 1H), 3.27-3.32 (q, 1H), 3.38-3.42 (q, 1H), 5.42 (m, 1H), 12.05 (s, 1H).
13C NMR (100 MHz, CDCl3): 23.46, 25.48, 28.38, 46.02, 46.59, 118.22, 123.91, 136.92, 161.73, 197.24, 205.66.
Lead Reference
Hsu, D.; Huang, J. J Org. Chem. 2012, 77, 2659-2666.
Other References
Fringuelli, F.; Pizzo, F.; Taticchi, A.; Halls, T. D. J.; Wenkert, E. J. Org. Chem. 1982, 47, 5056-5065.
Supplementary Information
Keywords
addition, aromatics/arenes, cycloaddition, Lewis-Acid mediated, regioselective
Comments
Additional Reference
Diels-Alder cycloaddition of juglone derivatives: elucidation of factors influencing regiochemical control
Robert K. Boeckman Jr., Terence M. Dolak, Kenneth O. Culos
J. Am. Chem. Soc., 1978, 100 (22), pp 7098–7100
DOI: 10.1021/ja00490a070
By Steven Merwin Kennedy on May 24, 2016