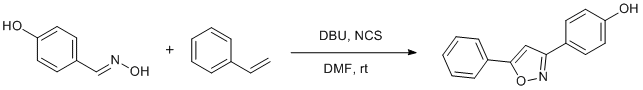
Metal-free DBU promoted cycloaddition of styrene to hydroxylimine
SyntheticPage 773
DOI:
Submitted: January 5, 2015, published: January 6, 2015
Authors
Sandip Bharate (sbharate@iiim.ac.in)
Shabber Mohammed (shabbiriiim@gmail.com)
A contribution from
Chemicals
Hydroxylamine hydrochloride (Sigma Aldrich), 99%, 159417
Sodium hydroxide (SD Fine Chemicals Ltd)
4-Hydroxybenzaldehyde (Sigma Aldrich), 98%, 144088
N-Chlorosuccinimide (Sigma Aldrich), 98%, 109681
1,8-Diazabicyclo[5.4.0]undec-7-ene (Sigma Aldrich), 98%, 139009
Dimethyl formamide (SD Fine Chemicals Ltd)Procedure
Preparation of 4-hydroxy phenylaldoxime: To the solution of hydroxylamine hydrochloride (1 g, 14.5 mmol) in water (10 ml) was added sodium hydroxide (307 mg, 7.6 mmol) and 4-hydroxy benzaldehyde (1.77 g, 14.5 mmol) and reaction mixture was stirred for 2 hrs at room temperature. After completion of the reaction (monitoring by TLC), if compound precipitated out, was filtered on Buckner funnel or extracted with EtOAc (3 ´ 15 ml). The obtained product was taken for next step without further purification (> 90% yield).
Procedure for synthesis of 3-(4-hydroxy phenyl) 5-phenyl-isoxazole: To the stirred solution of 4-hydroxy phenyl aldoxime (100 mg, 0.729 mmol) in DMF (3 ml) was added N-chlorosuccinimide (161 mg, 0.875 mmol) at room temperature and reaction was stirred for 0.5 h. Then, DBU (111 mg, 0.729 mmol) and styrene (91 mg, 0.875 mmol) were added and reaction was further stirred for 1 h. After completion of the reaction (confirmed by TLC), chilled water (20 ml) was added and product was extracted with EtOAc (3 ´ 10 ml). The organic layer was collected, dried on anhydrous sodium sulphate and solvent was evaporated on rotary evaporator to get the crude product. The crude product was purified by silica gel (#100-200) column chromatography using 20% EtOAc: hexane to get title compound as a white solid (138 mg; 80% yield).Author Comments
Data
3-(4-Hydroxy phenyl) 5-phenyl-isoxazole: White solid; m. p. 187-189 °C
1H NMR (400 MHz, CDCl3, ppm): δ 7.83 (dd, J = 4, 8 Hz, 2H), 7.76 (d, J = 8 Hz), 2H), 7.47 (m, 3H), 6.95 (d, J = 8 Hz, 2H), 6.78 (s, 1H).
13C NMR (CDCl3 + CD3OD, 125 MHz): δ 170.0, 162.9, 158.8, 130.2, 128.9, 128.2, 127.2, 125.7, 120.0, 115.7, 97.3.
IR (CHCl3): νmax 3433, 2925, 1631, 1450, 1353, 1095, 1017 cm-1.
ESI-MS: m/z 238.08 [M+H]+.
HRMS: m/z 238.0864 calcd for C15H11NO2+H+ (238.0864).
Lead Reference
Mohammed, S.; Vishwakarma, R.A.; Bharate, S.B. Metal-free DBU promoted regioselective synthesis of isoxazoles and isoxazolines. RSC Adv. 2015, 5, 3470-3473. http://dx.doi.org/10.1039/C4RA14694H
Other References
http://dx.doi.org/10.1021/ol401815n A. Yoshimura, C. Zhu, K. R. Middleton, A. D. Todora, B. J. Kastern, A. V. Maskaev and V. V. Zhdankin, Chem. Commun., 2013, 49, 4800-4802
http://dx.doi.org/10.1039/c3cc41164h B. A. Mendelsohn, S. Lee, S. Kim, F. Teyssier, V. S. Aulakh and M. A. Ciufolini, Org. Lett., 2009, 11, 1539-1542
http://dx.doi.org/10.1021/ol900194v A. M. Jawalekar, E. Reubsaet, F. P. J. T. Rutjes and F. L. van Delft, Chem. Commun., 2011, 47, 3198-3200
http://dx.doi.org/10.1039/c0cc04646a
Supplementary Information
Keywords
addition, DBU, heterocyclic compounds, Isoxazole, metal-free, regioselective