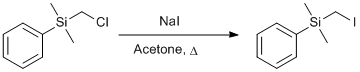
Finkelstein Reaction of (Chloromethyl)dimethyl(phenyl)silane with Sodium Iodide
SyntheticPage 758
DOI:
Submitted: July 11, 2014, published: August 11, 2014
Authors
Christopher Kelly (christopher.b.kelly@uconn.edu)
Leon Tilley (ltilley@stonehill.edu)
Michael Mercadante (michael.mercadante@uconn.edu)
Nicholas Leadbeater (nicholas.leadbeater@uconn.edu)
A contribution from
Chemicals
Sodium Iodide (ACS reagent, ≥99.5%, Sigma Aldrich)
Acetone (CHROMASOLV® Plus, ≥99.9%, Sigma Aldrich)
Procedure
In a 250 mL round bottom flask equipped with a stir bar was added (chloromethyl)dimethyl(phenyl)silane (20.13 g, 0.108 mol, 1 equiv), acetone (100 mL, 1.08 M) and sodium iodide (28.4 g, 0.189 mol, 1.75 equiv) and allowed to reflux for 24 h. After this time, the reaction was cooled to room temperature and the solvent removed via rotary evaporation to give a slurry. The resulting slurry was passed through a pad of Celite® which was washed with 100 mL of hexanes. The solvent removed via rotary evaporation to give the pure iodide as a clear, colorless liquid (30.1 g, 100%).
Author Comments
Data
1H NMR (400 MHz, CDCl3) d ppm 0.49 (s, 6 H) 2.22 (s, 2 H) 7.37 - 7.48 (m, 3 H) 7.54 - 7.63 (m, 2 H) 13C NMR (100 MHz, CDCl3) d ppm -13.25 (CH2) -2.59 (CH3) 128.25 (CH) 129.89 (CH) 133.94 (CH) 137.06 (C) GC-MS (EI) 276 ([M]+, 8%), 260 (20%), 149 (63%), 135 (100%), 119 (14%) 105 (17%), 92 (14%).
Lead Reference
Other References
Tilley, L. J.; Shiner, V. J., Jr. J. Phys. Org. Chem. 1999, 12, 564.
Keywords
Alkanes, nucleophilic, Organosilicon, SN2, substitution