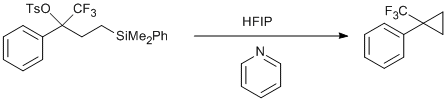
Solvolysis of 4-(dimethyl(phenyl)silyl)-1,1,1-trifluoro-2-phenylbutan-2-yl 4-methylbenzenesulfonate
SyntheticPage 756
DOI:
Submitted: July 11, 2014, published: August 11, 2014
Authors
Christopher Kelly (christopher.b.kelly@uconn.edu)
Leon Tilley (ltilley@stonehill.edu)
Michael Mercadante (michael.mercadante@uconn.edu)
Nicholas Leadbeater (nicholas.leadbeater@uconn.edu)
A contribution from
Chemicals
Hexafluoroisopropanol, HFIP (99%, Synquest Laboratories)
Pyridine (ACS reagent, ≥99.0%, Sigma Aldrich)
Procedure
To a 25 mL screw top vial equipped with a stir bar was added HFIP (10 mL) and pyridine (1.58 g, 0.020 mol, 2 equiv) (CAUTION: mildly exothermic). The mixture was allowed to stir at room temperature for approximately five minutes. At this time the solution was poured into added to another 25 mL screw top vial equipped with a stir bar containing the toyslate (4.93 g, 10.0 mmol) (CAUTION: mildly exothermic) and the vial was sealed. The biphasic reaction mixture was stirred at room temperature. Once the reaction was judged complete1,2, the reaction mixture was transferred to a separatory funnel and diluted with pentane (≈ 100 mL) and deionized water (≈ 150 mL). The layers were separated and the aqueous layer was extracted twice with pentane (≈ 100 mL). The combined organic layers were washed with deionized water twice (≈ 100 mL) and one with brine (≈ 100 mL). The organic layer was dried with Na2SO4 and the solvent was removed in vacuo via rotary evaporation (50 mmHg, 30 oC water bath)3. Further purification was accomplished by flash column chromatography (hexanes) to give the pure CF3 cyclopropane as a clear, colorless oil (1.07 g, 58%).
Author Comments
2. Note that, it is likely that these reaction are completed after the solid material dissolves (<1 hr) due to the rapidity of ring closure and the strong solvolytic power of HFIP. However, it is recommended that the reaction is monitored by NMR to determine reaction progress. Other forms of reactions monitoring (TLC or GC/MS) proved ineffective for determining progress due to decomposition of the OTs starting material.
3.Note it is imperative that higher pressures are used during rotary evaporation to ensure reasonable yield. This CF3 cyclopropane is particularly volatile and can easily be lost during solvent removal at lower pressures
Data
Lead Reference
Other References
Keywords
Alkanes, aromatics/arenes, Cyclopropanes, elimination, Organosilicon, Solvolysis, Trifluoromethyl