Olefination of 3-phenyl-6-bromoindene
SyntheticPage 697
DOI:
Submitted: October 1, 2013, published: October 4, 2013
Authors
Adam Glass (glassac@plu.edu)
Valerie Lesniak (lesniava@plu.edu)
A contribution from
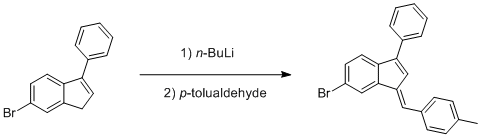
Chemicals
Indene 1 (Prepared in House by method of SP696)
n-BuLi (2.5 M solution in hexanes, Acros Organics)
p-tolualdehyde (TCI)
2-MeTHF (≥99%, Anhydrous, Sigma-Aldrich)
Diethyl Ether (Sigma-Aldrich)
Dichloromethane (Sigma-Aldrich)
Saturated Ammonium Chloride
Procedure
Procedure A: The synthesis was performed using standard Schlenk techniques under a nitrogen atmosphere. To a sealed scintillation vial charged with indene 1 (43.7 mg, 0.161 mmol), anhydrous 2-MeTHF (1.6 mL) was added. The solution was cooled to 0 oC and n-BuLi (0.070 mL, 0.161 mmol, 2.5 M solution in hexanes) was added dropwise. The reaction mixture was then stirred for 1 h. p-tolualdehyde (0.020 mL, 0.169 mmol) was added to the reaction mixture at 0 oC. The reaction mixture was returned to RT and stirred for 20 h. TLC was used to monitor the reaction.* The reaction mixture was quenched and washed with saturated ammonium chloride (3 x 1 mL) and extracted with diethyl ether (3 x 1 mL). The organic layer was dried over magnesium sulfate, filtered into a roundbottom flask, and further eluted with diethyl ether (3 mL). The solvent was evaporated under reduced pressure. The crude product was dissolved in dichloromethane and purified using a preparatory plate (90:10, hexanes:toluene), yielding a light red solid, benzofulvene 2 (28.7 mg, 47%).
Procedure B: The synthesis was performed using standard Schlenk techniques under a nitrogen atmosphere. To a sealed scintillation vial charged with indene 1 (100 mg, 0.369 mmol), anhydrous heptane (3.7 mL) was added. The solution was cooled to 0 oC and n-BuLi (0.15 mL, 0.369 mmol, 2.5 M solution in hexanes) was added dropwise. The reaction mixture was warmed to RT and stirred overnight. A tan precipitate develops. The heptanes was removed under reduced pressure and anhydrous 2-MeTHF (3.7 mL) was added. The solution was cooled to 0 oC and p-tolualdehyde (0.048 mL, 0.406 mmol) was added dropwise. The reaction mixture was returned to RT and stirred for 20 h. TLC was used to monitor the reaction.* The reaction mixture was quenched and washed with saturated ammonium chloride (3 x 2 mL) and extracted with diethyl ether (3 x 2 mL). The organic layer was dried over magnesium sulfate, filtered into a roundbottom flask, and further eluted with diethyl ether (3 mL). The solvent was evaporated under reduced pressure. The crude product was dissolved in dichloromethane and purified using a preparatory plate (90:10, hexanes:toluene), yielding a light red solid, benzofulvene 2 (81.0 mg, 59%).
*An aliquot was removed from the crude reaction mixture and diluted for TLC.
Author Comments
Butyl lithium is pyrophoric. It should be handled under inert conditions.
The synthesis using heptanes as the solvent for the lithiation step (Procedure B) was determined to be more reproducible. Arguably due to acid/base chemistry between n-BuLi and 2-MeTHF.
Benzofulvenes are highly colored molecules. Chromatography is a excellent route for purification as the desired compound can be observed directly during purification.
A saturated solution of sodium chloride (Brine) was used to remove emulsions during the washing phases.
Data
1H NMR (CDCl3, 500 MHz): δ ppm 2.54 (s, 3H), 7.27 (s, 1H), 7.38-7.39 (m, 2H), 7.51-7.61 (m, 6H), 7.69 (d, J = 8.3 Hz, 2H), 7.77-7.79 (m, 2H), 8.00 (s, 1H)
13C NMR (CDCl3, 125 MHz): δ ppm 21.66, 119.76, 121.27, 122.86, 123.67, 128.54, 128.54, 128.97, 129.88, 129.94, 129.98, 130.60, 134.15, 135.61, 137.57, 139.38, 139.57, 141.25, 146.72
GC-MS EI [M.+]: Predicted: 372.05, Actual: 372; Predicted: 374.06, Actual: 374; Predicted: 373.05, Actual: 373
Lead Reference
Other References
Lu, W.; Zhu, Q.; Yan, C. Synth. Commun., 1997, 27, 3985-3990.
Alcalde, E.; Mesquida, N.; Frigola, J.; López-Pérez, S.; Mercè, R. Org. Biomol. Chem., 2008, 6, 3795-3810Keywords
aldehydes, aromatics/arenes, Li, nucleophilic, organometallics