Oxidation of furfuryl alcohols
SyntheticPage 65
DOI:
Submitted: August 15, 2001, published: August 16, 2001
Authors
lisa frost (lisa.frost@evotec.com)
A contribution from
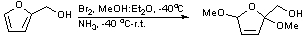
Chemicals
Bromine (Aldrich)
methanol
furfuryl alcohol (distilled)
Ether (distilled from sodium benzophenone ketyl)
methanol
furfuryl alcohol (distilled)
Ether (distilled from sodium benzophenone ketyl)
Procedure
A solution of bromine (136.9 g, 0.86 mol) in methanol (239 mL) was slowly added via addition funnel to a stirred solution of furfuryl alcohol (70.4 g, 0.718 mol) in dry diethyl ether (239 mL) and methanol (239 mL) maintaining an internal temperature between –35 oC and –45 oC. Upon addition completion, stirring was continued for a further 2 hours at reduced temperature. The resulting light yellow solution was saturated with gaseous ammonia to pH 8 and allowed to warm to an ambient temperature. The resulting yellow suspension was filtered, removing ammonium bromide and concentrated in vacuo. Further filtration removed the white solid formed upon concentration of the yellow oil. The oil was diluted in benzene (500 mL) and filtered through neutral alumina. Evaporation of the solvent yielded a yellow oil (102.6 g, 90.4 %) which was seen to be of sufficient purity further reactivity
Author Comments
This reaction has been carried out on numerous occasions by both experienced and non-experienced chemists. Although we usually do this reaction on a large scale (typically 70 g) it is equally successful on a small scale. One thing to note is that the internal temperature of the reaction can rise very quickly so be careful.
TIP: jacket your addition funnel and force cold air through the jacket as this keeps the bromine solution cold and slows down the warming process.
When adding ammonia gas to the solution it is important to retain a low temperature (-40oC) and add ammonia until a clear solution is afforded
Data
1H nmr (300 MHz, CDCl3) 1.95 (1H, br s), 3.09 (3H, s), 3.38 (3H, s), 3.52-3.69 (2H, m), 5.48 (1H, d J 0.9), 5.93 (1H, d J 4.7), 6.04 (1H, d J 4.7); 13C nmr (75 MHz, CDCl3) 50.4, 56.2, 66.6, 107.3, 112.9, 131.3, 132.2.
Lead Reference
Achmatowicz Jr. O, Bukowski P, Szechner B, Zwierzchowska Z, Zamoiski A,
Tetrahedron 1971, 27, 1973-1996.
Other References
Caddick S, Khan S, Frost LM, Smith NJ, Cheung S, Pairaudeau G, Tetrahedron, 2000, 56 (45) 8953-8958