Amidation of a pyridine acylchloride with phenylglycine
SyntheticPage 637
DOI:
Submitted: July 19, 2013, published: July 22, 2013
Authors
Nikola P. Chmel (n.chmel@warwick.ac.uk)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
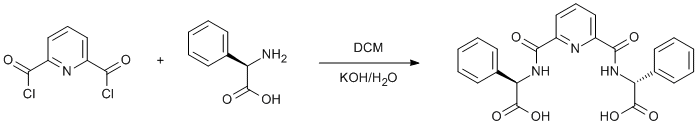
Chemicals
KOH
Pyridine-2,6-dicarbonyl dichloride (Prepared in house, see page 633)
Procedure
(R)-2-phenylglycine (1.53 g, 10.2 mmol) was suspended in water (25 ml) and solid KOH was added until the solution cleared. The mixture was chilled in an ice/water bath and pyridine-2,6-dicarbonyl chloride (1.04 g, 5.1 mmol) in DCM (25 ml) was added. The mixture was stirred for 0.5 h in an ice/water bath, then for another 0.5 h at ambient temperature. The DCM layer was discarded. The aqueous layer was diluted with a further 100 ml of water and was acidified with formic acid (6 ml of conc. HCOOH in 30 ml of water) until turbidity developed. The solution was left to crystallise overnight. The product was filtered, washed successively with water, ethanol and diethyl ether and dried in vacuo. The filtrate was concentrated to obtain the second crop. The combined product was recrystallised from methanol/water. Yield 0.55 g (25%).
Author Comments
Data
Elemental analysis found (calculated for C23H19N3O6∙3H2O) %: C 56.91 (56.67), H 5.17 (5.17), N 8.55 (8.62).
1H NMR (400 MHz, 298 K, CDCl3) δH 5.71 (s, 2H, CH), 7.31-7.43 (m, 6H, Ph-H), 7.54 (d, 4H, JHH=8Hz, Ph-H), 8.13 (t, 1H, JHH=8Hz, pyr), 8.26 (d, 2H, JHH=8Hz)
13C NMR (100 MHz, 298 K, CDCl3) δC 58.2 (CH), 126.4, 128.7, 129.5, 130.0 (Ph), 138.0, 140.7, 150.0 (pyr), 165.3 (C=O), 173.6 (COOH)
MS (ESI+) m/z 456.12 [(M+Na]+), 889.24 ([2M+Na]+)
IR (cm-1) ν 3391, 3036, 1725, 1669, 1513, 1442, 1389, 1319, 1253, 1182, 1095, 1074, 1030, 1001, 967, 843, 749, 724, 695, 663
Lead Reference
http://dx.doi.org/10.1021/ja00299a038
Other References
Keywords
acylation, acylchloride, addition, amines, elimination