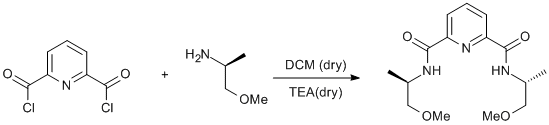
Amidation of a pyridine acylchloride using 1-methoxy-2-propylamine
SyntheticPage 636
DOI:
Submitted: July 19, 2013, published: July 22, 2013
Authors
Nikola P. Chmel (n.chmel@warwick.ac.uk)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
Chemicals
S-(+)-1-methoxy-2-propylamine (Aldrich)
Dichloromethane (distilled over potassium)
Triethylamine (distilled over calcium hydride)
Procedure
Pyridine-2,6-dicarbonyl dichloride (1.0 g, 4.8 mmol) was dissolved in dry DCM (20 ml) under argon. S-(+)-1-methoxy-2-propylamine (1.08 ml, 10.2 mmol) was added dropwise and the mixture was stirred for 15 min. Dry TEA (~6 ml) was added and the mixture was stirred at ambient temperature overnight. The mixture was then washed with water (3 × 20 ml) and dried over sodium sulphate. The solvent was removed under reduced pressure and the residue was recrystallised from DCM/pentane. Yield 1.2 g (78%).
Author Comments
Data
Elemental Analysis found (Calculated for C15H23N3O4) %: C 58.45 (58.24), H 7.60 (7.49), N 13.64 (13.58)
1H NMR (400 MHz, 298 K, CDCl3) δH 1.33 (6H, d, 3JHH = 6.8 Hz, -CH3), 3.42 (6H, s, -OCH3), 3.46-3.56 (4H, m, CH2), 4.29-4.42 (2H, m, CH), 7.92-8.05 (3H, m (s+t), NH, py), 8.32 (2H, d, 3JHH = 7.8 Hz, pyr)
13C NMR (100 MHz, 298 K, CDCl3) δC 17.7 (-CH3), 45.0 (CH), 59.1 (-OCH3), 75.4 (CH2), 124.8, 138.9, 148.8 (py), 162.8 (C=O)
MS (ESI+) m/z 332.1 ([M+Na]+); (ESI-) m/z 308.1 ([M-H]-)
IR (cm-1) ν 3327, 2974, 2930, 2879, 2831, 1651, 1519, 1440, 1393, 1365, 1353, 1325, 1303, 1255, 1230, 1203, 1173, 1150, 1108, 1080, 1062, 997, 978, 925, 847, 758, 726
Lead Reference
Keywords
acyl chloride, addition, amides, amines, aromatics/arenes