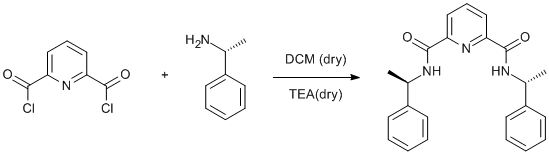
Amidation of a pyridine acylchloride using α-methylbenzylamine
SyntheticPage 635
DOI:
Submitted: July 19, 2013, published: July 22, 2013
Authors
Nikola P. Chmel (n.chmel@warwick.ac.uk)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
Chemicals
(R)-(+)-α-methylbenzylamine (Aldrich)
Dichloromethane (distilled over potassium)
Triethylamine (distilled over calcium hydride)
Procedure
Pyridine-2,6-dicarbonyl dichloride (2.04 g, 10 mmol) was dissolved in dry DCM (50 ml). (R)-(+)-a-methylbenzylamine (2.42g, 2.54 ml, 20mmol) and dry triethylamine (2.02g, 2.8 ml, 20mmol) were added dropwise and the solution was stirred for 2 h. The reaction mixture was then washed with water (3×50 ml) and dried over MgSO4. The solvent was removed under reduced pressure and the obtained solid was recrystallised from DCM/pentane. Yield 2.7 g (72%).
Author Comments
Data
Elemental Analysis found (Calculated for C23H23N3O2) %: C 73.63 (73.97), H 6.50 (6.21), N 10.99 (11.25).
1H NMR (300 MHz, CDCl3) δ 8.35 (d, J = 7.8 Hz, 2H), 8.03 (t, 1H), 7.81 (d, J = 8.2 Hz, 2H), 7.43 – 7.30 (m, 10H), 5.31 (q, J = 6.9 Hz, 2H), 1.61 (d, J = 6.9 Hz, 6H).
13C NMR (100 MHz, 298 K, CDCl3) δC 21.9 (CH3), 49.1 (C-H), 125.1 (ph), 126.1, 127.6, 128.9, 139.1 (pyr), 143.0, 148.8, 162.6 (C=O)
MS (ESI+)m/z 396.17 ([M+Na]+)
IR (cm-1) ν 3610, 3395 (N-H), 1713, 1663 (C=O), 1508 (N-H), 1445 (C-C ar.),1256, 1183, 1100, 1073 (C-H ar.), 1000, 840, 698 (C-H ar)
Lead Reference
Keywords
acyl chloride, addition, amidation, amides, amines