BOC protection and methylsulfonylation of phenylglycinol
SyntheticPage 607
DOI:
Submitted: July 9, 2013, published: July 11, 2013
Authors
Nikola P. Chmel (N.Chmel@warwick.ac.uk)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
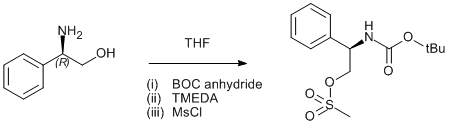
Chemicals
(R)-phenylglycinol(Prepared in house, see Page 275)
TMEDA
Methanesulfonyl chloride (Fluka)
Procedure
BOC anhydride (1.68 g, 7.7 mmol) in THF (5 ml) was added dropwise to a solution of (R)-phenylglycinol Page 275 (1.0 g, 7.3 mmol) and was stirred for 30 min. TMEDA (0.90 g, 7.7 mmol) was added and the mixture was cooled in an ice/water bath. Methanesulfonyl chloride (0.89 g, 7.7 mmol) was added and the mixture was stirred for 1.5 h. The solids were removed by filtration and washed with THF. Hexanes were added to the combined filtrates to initiate the crystallisation. The product was collected by filtration, washed with hexanes and dried in air. Yield 2.0 g (87%).
Author Comments
Data
1H NMR (400 MHz, 298 K, CDCl3) δH 7.42 – 7.28 (m, 5H, Ph), 5.25 – 5.09 (m, 1H, NH), 5.08 – 4.94 (m, 1H, CH), 4.55 – 4.33 (m, 2H, CH2), 2.88 (s, 3H, SO3CH3), 1.43 (s, 9H, tBu).
13C NMR (100 MHz, 298 K, CDCl3) δC 137.8, 129.09, 128.47, 126.82 (Ph), 71.38 (CH2), 53.7 (CH), 37.61 (SO3CH3), 28.44 (C(CH3)3)
MS (ESI+) m/z 338.0 ([M+Na]+)
IR (cm-1) ν 3357, 2982, 2939, 1693, 1522, 1497, 1456, 1391, 1356, 1329, 1277, 1252, 1163, 1099, 1079, 1053, 1028, 1002, 959, 923, 886, 851, 815, 758, 702, 653.
Lead Reference
Keywords
addition, alcohols, amines, BOC, mesylate, methanesulfonylation, protecting group