Addition of a primary amine to cyclohexyl isothiocyanate
SyntheticPage 601
DOI:
Submitted: July 8, 2013, published: July 10, 2013
Authors
Edward J Crust (e.j.crust@warwick.ac.uk)
Pratik Gurnani (pratik.gurnani@warwick.ac.uk)
A contribution from
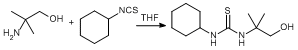
Chemicals
cyclohexyl isothiocyanate (Aldrich)
THF (distilled over potassium)
Procedure
To a Schlenk vessel charged with 2-amino-2-methyl-1-propanol (6.6 g, 74.1 mmol) in dry THF (20 mL) was added cyclohexyl isothiocyanate 4 (5.0 g, 35.4 mmol) in THF (10 mL). The solution was then stirred overnight to yield an off-white precipitate. The precipitate was then isolated by filtration and washed with cold chloroform. Any residual solvent was then removed by evaporation under reduced pressure (yield 6.92 g, 85 %).
Author Comments
Data
1H NMR (293 K, d5-pyridine) d 1.06 (m, 2H, cyclohexyl-CH2), 1.36 (m, 2H, cyclohexyl-CH2), 1.47 (s, 6H, CH3), 1.59 (m, 2H, cyclohexyl-CH2), 2.15 (m, 2H, cyclohexyl-CH2), 3.80 (s, 2H, CH2), 4.62 (m, 2H, cyclohexyl-CH), 5.07 (s, 1H, OH), 7.95 (br s, 1H, NH), 9.26 (br s, 1H, NH).
13C{1H} NMR (293 K d5-pyridine) d 26.6 (CH3), 26.9 (cyclohexyl-CH2), 27.9 (cyclohexyl-CH2), 34.9 (cyclohexyl-CH2), 55.9 (cyclohexyl-CH), 59.3 (Me2Cq), 72.8 (CH2), 183.7 (NCqN).
IR (Thin film, cm-1): 3225, 3072, 2995, 2975, 2927, 2852, 1599, 1530, 1447, 1424, 1370, 1346, 1303, 1270, 1259, 1234, 1189, 1167, 1152, 1114, 1054, 986, 888, 854, 820, 787, 758, 673.
Anal. Calcd. for C11H22N2SO: C, 57.35; H, 9.63; N, 12.16. Found: C, 57.26; H, 9.59; N, 12.17.
MS (EI) m/z 230 (M+)
Lead Reference
E. J. Crust, I. J. Munslow and P. Scott, Journal of Organometallic Chemistry, 2005, 690, 3373-3382.http://dx.doi.org/10.1016/j.jorganchem.2005.04.019
Keywords
2-amino-2-methyl-1-propanol, addition, alcohols, amines, cyclohexyl isothiocyanate, thiourea