Dehydration of N-formylamino acid esters with Triphosgene
SyntheticPage 591
DOI:
Submitted: March 13, 2013, published: March 15, 2013
Authors
Daniel Carney (daniel_carney@brown.edu)
A contribution from
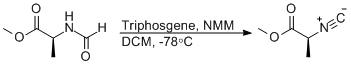
Chemicals
Triphosgene (98%, TCI)
N-methylmorpholine (99%, Alfa Aesar)
Dichloromethane (anhydrous)
Sodium Sulfate (Fisher)
Procedure
N-formylalanine methyl ester (2.07 g, 15.8 mmol) was added to an oven dried 100 mL round bottom flask with a magnetic stirring bar and sealed with a septum. The flask was then blow-dried under vacuum and subsequently purged with dry nitrogen. Once cooled to room temperature, dichloromethane (40 mL) was added to the flask, dissolving the N-formylalanine methyl ester. The flask was then suspended in a dry ice / acetone bath and allowed to cool for 10 minutes. Meanwhile, triphosgene1 (1.64 g, 5.53 mmol) was added to an oven dried round bottom flask and sealed with a septum. The flask was purged with dry nitrogen and then dichloromethane (10 mL) was added, dissolving the triphosgene. The triphosgene solution was then transferred into the cold N-formylalanine methyl ester solution via syringe. The reagents were allowed to mix for 5 minutes after which N-methylmorpholine (3.49 mL, 31.62 mmol) was added drop-wise over the course of 5 minutes.2 After 1 hour and 45 minutes3 the reaction flask was removed from the dry ice / acetone bath and immediately quenched with water (25 mL), which instantaneously turned to ice.4 Once the water had, melted the reaction solution was transferred to a separatory funnel and the phases separated. The aqueous layer was subsequently washed three times with dichloromethane. The combined organic layers containing the isocyanoacetate product were dried over sodium sulfate. The dried Isocyanoacetae solution was then filtered through a 2 inch plug of silica gel, rinsing with an additional 50 mL of dichloromethane.5 The solvent was removed by rotary evaporation at 30°C providing pure isocyanoacetate product.6 Yield 1.41 g, 79%.
Author Comments
- Triphosgene is slightly moisture sensitive and highly toxic. This reagent must be handled with care. Neither the triphosgene bottle nor the flask used to dissolve the triphosgene were ever opened outside of the chemical fume hood
- Danishefsky et al. have reported a similar procedure wherein the N-methylmorpholine is premixed with the N-formylalanine methylester and the reaction is initiated by rapid addition of the triphosgene solution. I have tested both methods and they seem to work equally as well.
- Consumption of the N-formylalanine methyl ester starting material can be monitored by TLC (Rf ~0.3, 20% ethyl acetate / 80% hexanes, visualization with permanganate stain)
- Upon completion of the reaction, the color of the solution varies from colorless to pale yellow. Ideally the reaction should develop no color. It is okay if the reaction turns yellow. However, you may obtain a lower than expected yield. Ensuring that the N-formylalanine methyl ester starting material is highly pure and solvent free is key to executing an ideal dehydration.
- If the crude product solution is yellow, the filtration through silica gel will produce a colorless filtrate.
- Isocyanoacetates are somewhat volatile and have a pungent odor. To remove the last bit of dichloromethane solvent, I recommend blowing the product with a stream of nitrogen in the chemical fume hood.
General Remarks
- I have also used this procedure to prepare isocyanoacetates derived from leucine methyl ester, valine methyl ester, phenylalanine methyl ester, and methionine methyl ester.
- Isocyanoacetates decompose when neat at room temperature. I recommend using them in subsequent reactions soon after their preparation. If you do need to store them, I recommend storing them as a dichloromethane solution at -20°C.
- Alpha-substituted isocyanoacetates, such as those derived from alpha-amino acid esters, are configurationally unstable, which is another reason to use them soon after preparation. Despite some controversy in the literature, I have executed dozens of multicomponent reactions (Passerini, Ugi, Joullié-Ugi) with minimal epimerization at the isocyanoacetate alpha-carbon.
Data
Lead Reference
Other References
Zhu, J.; Wu, X.; Danishefsky, S. J. Tetrahedron Lett. 2009, 50, 577.
Keywords
amino acids, dehydration, isocyanide, isocyanoacetate, triphosgene