N-Oxidation of 5-hydroxy-2-methylpyridine
SyntheticPage 546
DOI:
Submitted: March 26, 2012, published: March 29, 2012
Authors
Rebecca Kaner (r.a.kaner@warwick.ac.uk)
A contribution from
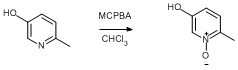
Chemicals
5-hydroxy-2-methylpyridine
Chloroform
Ethyl Acetate
Procedure
3-Chloroperoxybenzoic acid (77%, 58.04 g, 259 mmol, 1.1 eq.) was added slowly as a solid to a suspension of 5-hydroxy-2-methylpyridine (25.44 g, 233 mmol) in chloroform (600 ml). After all the solid had dissolved to leave a clear yellow solution, the reaction was stirred at reflux (75°C) for 2 h. After this time the solution was allowed to cool to ambient temperature before the solvent was removed under reduced pressure. The crude yellow solid was washed with hot ethyl acetate (3 × 300 ml) and dried overnight in vacuo. Yield = 22.33 g, 77%.
Author Comments
- Reaction can be carried out on smaller or larger scale with relative ease
- Add mCPBA slowly (exothermic reaction)
Data
1H NMR (400 MHz, 298 K, DMSO) δH 10.24 (1H, br s, OH), 7.81 (1H, d, 4JHH = 2.0 Hz, Py), 7.24 (1H, d, 3JHH = 8.5 Hz, Py), 6.77 (1H, dd, 3JHH = 8.5 Hz , 4JHH = 2.0 Hz, Py), 2.22 (3H, s, CH3).
13C{1H} NMR (100 MHz, 298K, DMSO) δC 153.9 (Py), 138.9 (Py), 127.3 (Py), 126.0 (Py), 113.7 (Py), 16.2 (CH3).
MS (ESI) m/z 126.3 [M+H]+, 148.2 [M+Na]+.
IR v cm-1 2215 w, 1622 w, 1570 w, 1529 m, 1459 m, 1422 m, 1386 m, 1309 m, 1275 w, 1226 w, 1663 m, 1117 m, 1001 m, 961 m, 887 m, 863 s, 825 s, 775 m, 740 s, 692 m.
Elemental Analysis found (Calculated for C6H7NO2) % C 57.57 (57.59), H 5.62 (5.64), N 11.05 (11.19).
Lead Reference
Supplementary Information
Keywords
heterocyclic compounds, N-Oxidation, oxidation