Dehydration of 3,4-dihydro-5H-Benzo[cd]pyren-5-ol
SyntheticPage 542
DOI:
Submitted: March 15, 2012, published: May 31, 2012
Authors
Anish Mistry (a.mistry@warwick.ac.uk)
A contribution from
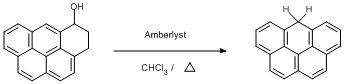
Chemicals
3,4-dihydro-5H-Benzo[cd]pyren-5-ol (prepared)
Amberlyst 15 (Sigma-Aldrich)
Chloroform
Amberlyst 15 (Sigma-Aldrich)
Chloroform
Procedure
3,4-dihydro-5H-Benzo[cd]pyren-5-ol (0.1 g, 0.39 mmol) was dissolved in chloroform (30 ml) and Aberlyst 15 (0.1 g) added under a dinitrogen atmosphere. The reaction was heated to 30oC and left overnight under the inert atmosphere. The solution was then filtered to seperate the Amberlyst and washed with chloroform. The combined solvents were removed under vacuum using a Rotary evaporator. The crude product was column chromatographed under a dinitrogen atmosphere eluting with 1:1 chloroform:petroleum ether 40-60oC. A white solid was obtained using this method (50 mg, 54%).
Author Comments
- Degradation of product occurs on the column. The use of a dinitrogen atmosphere slows this down. Firstly the eluent and column were purged using dinitrogen gas, after the crude mixture was loaded on the purged column a continous flow of dinitrogen gas was used for flash chromatography via a nitrogen line.
- The chloroform was washed with sodium bicarbonate before column chromatography.
- The white product on exposure to air became yellow then orange in colour.
Data
δH(400MHz, CDCl3) ppm: 5.00 (2H, s, -CH2), 7.46 - 7.55 (4H, m, aryl), 7.72 - 7.84 (6H, m, aryl).
Supplementary Information
Keywords
aromatics/arenes, dehydration