N-alkylation of pyridine with acetyl compound;
SyntheticPage 541
DOI:
Submitted: March 14, 2012, published: March 14, 2012
Authors
Suzanne Elizabeth Howson (s.e.howson@warwick.ac.uk)
A contribution from
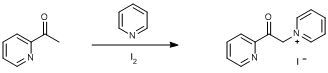
Chemicals
2-Acetylpyridine (Sigma-Aldrich)
Iodine (Fisher Scientific)
Activated charcoal (Sigma-Aldrich)
Dry pyridine - dried by heating to reflux for 3 d under dinitrogen over calcium hydride and degassed before use. Stored in glass ampoules under argon.
Diethyl ether (Fisher Scientific)
Ethanol (Fisher Scientific)
Methanol (Fisher Scientific)
Iodine (Fisher Scientific)
Activated charcoal (Sigma-Aldrich)
Dry pyridine - dried by heating to reflux for 3 d under dinitrogen over calcium hydride and degassed before use. Stored in glass ampoules under argon.
Diethyl ether (Fisher Scientific)
Ethanol (Fisher Scientific)
Methanol (Fisher Scientific)
Procedure
2-Acetylpyridine (6.05 g, 5.6 ml, 50 mmol) was added via syringe to a solution of iodine (12.69 g, 50 mmol) in dry pyridine (50 ml) in a 250 ml round bottomed Schlenk vessel. The round bottomed Schlenk was fitted with a condenser and a N2 bubbler, and the reaction was stirred and heated at reflux (130oC) for 2 h. The solution was then cooled to ambient temperature. A 9:1 mixture of diethyl ether/ethanol (20 ml) was added and the solution was cooled to 0oC using an ice/water bath. The resulting black precipitate was filtered off, washed with a 9:1 mixture of diethyl ether/ethanol (20 ml), and dried in air. The solid was then dissolved in boiling methanol (200 ml) with activated charcoal (20 g) and stirred at reflux for 10 min. The solution was filtered through celite in a fritted funnel and the solvent was removed under reduced pressure to leave the crude product. Recrystallisation from hot methanol (100 ml) resulted in light brown crystals which were filtered, washed with cold methanol (25 ml), and dried in vacuo. Yield = 6.07 g, 18.61 mmol, 37%.
Author Comments
Yield can be increased by reducing the volume of the recrystallisation filtrate and leaving this solution in the fridge.
Data
1H NMR (400 MHz, 298 K, DMSO) δH 9.02 (2H, dd, 3JHH = 6.5 Hz, 4JHH = 1.0 Hz, Py), 8.87 (1H, dt, 3JHH = 4.5 Hz, 4JHH = 1.0 Hz, Py), 8.73 (1H, tt, 3JHH = 8.0 Hz, 4JHH = 1.5 Hz, Py), 8.28 (2H, m, Py), 8.14 (1H, td, 3JHH = 7.5 Hz, 4JHH = 1.5 Hz, Py), 8.07 (1H, dt, 3JHH = 8.0 Hz, 4JHH = 1.0 Hz, Py), 7.84 (1H, m, Py), 6.51 (2H, s, CH2).
13C{1H} NMR (100 MHz, 298 K, DMSO) δC 191.49 (C=O), 150.46 (Py), 149.58 (Py), 146.36 (Py), 146.31 (Py), 138.17 (Py), 129.16 (Py), 127.73 (Py), 122.06 (Py), 66.66 (CH2).
MS (ESI) m/z 199.1 [M+].
13C{1H} NMR (100 MHz, 298 K, DMSO) δC 191.49 (C=O), 150.46 (Py), 149.58 (Py), 146.36 (Py), 146.31 (Py), 138.17 (Py), 129.16 (Py), 127.73 (Py), 122.06 (Py), 66.66 (CH2).
MS (ESI) m/z 199.1 [M+].
Lead Reference
Eur. J. Inorg. Chem., 2011, 20, 3050-3058 (supporting information)
Keywords
addition, heterocyclic compounds, ketones, nucleophilic, Pyridinium iodide salt, substitution