Bromination of an amide enolate
SyntheticPage 54
DOI:
Submitted: August 15, 2001, published: August 15, 2001
Authors
Mala Mistry (m.mistry@sussex.ac.uk)
A contribution from
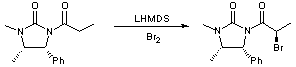
Chemicals
LHMDS (1M in Hexane) (Aldrich)
Bromine (Aldrich)
THF (Distilled from sodium benzophenone)
Bromine (Aldrich)
THF (Distilled from sodium benzophenone)
Procedure
A solution of the amide (1g, 4.06 mmol, 1 eq) in THF (10 mL) was cooled to -78oC, to which was added LHMDS (4.47 mL, 4.47 mmol, 1.15 eq) dropwise. The pale yellow solution was stirred for 40 minutes at this temperature before the bromine (0.24 mL, 4.46 mmol, 1.1 eq) was added dropwise. After 10 minutes of stirring the brown solution, the reaction was quenched by the addition of sat. NH4Cl solution (10 mL). The mixture was diluted with EtOAc (10 mL) and washed with sat. NH4Cl solution (4 x 10 mL) and brine (2 x 5 mL). The organic layer was dried with MgSO4. The crude product was purified by recrystallisation in EtOAc to afford a white solid (0.79 g, 60 %).
Author Comments
This reaction is a particularly efficient way of introducing a bromine in the alpha position. This step has been repeated many times and always afforded good yields >60 % and as an easy to handle white solid. The scale of the reaction has been carried out from using 10 mg to 1 g and always giving positive results.
This particular reaction protocol can be applied to different chains ranging from the stated propionyl chain to 6-phenyl hexanoyl chain, all being attached to the chiral auxiliary. However some difficulties have arisen from the bromination of a straight alkyl heptanoyl chain.
Concerning the stereochemistry of this reaction, I mainly observed the 2'R stereoisomer (>90 %)using this particular chain. But it has been observed that the ratio of the diastereomers depends on the different chains coupled to the chiral auxiliary.
Data
1H NMR (300 MHz, CDCl3) 0.92 (3H, d, J&, CCH3) 1.67 (3H, d, J5, CH2CH3) 2.97 (3H, s, NCH3) 4.00-4.09 (1H, m, CHCH3) 5.46 (1H, d, J9, CHPh) 6.06-6.12 (1H, m, CHCH2) 7.01-7.23 (5H, m, CHar)
Lead Reference
NONE
Other References
NONE